A review of the effects of unilateral hearing loss on spatial hearing
- PMID: 30143248
- PMCID: PMC6341410
- DOI: 10.1016/j.heares.2018.08.003
A review of the effects of unilateral hearing loss on spatial hearing
Abstract
The capacity of the auditory system to extract spatial information relies principally on the detection and interpretation of binaural cues, i.e., differences in the time of arrival or level of the sound between the two ears. In this review, we consider the effects of unilateral or asymmetric hearing loss on spatial hearing, with a focus on the adaptive changes in the brain that may help to compensate for an imbalance in input between the ears. Unilateral hearing loss during development weakens the brain's representation of the deprived ear, and this may outlast the restoration of function in that ear and therefore impair performance on tasks such as sound localization and spatial release from masking that rely on binaural processing. However, loss of hearing in one ear also triggers a reweighting of the cues used for sound localization, resulting in increased dependence on the spectral cues provided by the other ear for localization in azimuth, as well as adjustments in binaural sensitivity that help to offset the imbalance in inputs between the two ears. These adaptive strategies enable the developing auditory system to compensate to a large degree for asymmetric hearing loss, thereby maintaining accurate sound localization. They can also be leveraged by training following hearing loss in adulthood. Although further research is needed to determine whether this plasticity can generalize to more realistic listening conditions and to other tasks, such as spatial unmasking, the capacity of the auditory system to undergo these adaptive changes has important implications for rehabilitation strategies in the hearing impaired.
Keywords: Auditory cortex; Binaural; Monaural spectral cue; Plasticity; Sound localization; Spatial release from masking.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures

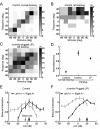
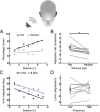
References
-
- Agterberg M.J., Snik A.F., Hol M.K., Van Wanrooij M.M., Van Opstal A.J. Contribution of monaural and binaural cues to sound localization in listeners with acquired unilateral conductive hearing loss: improved directional hearing with a bone-conduction device. Hear. Res. 2012;286:9–18. - PubMed
-
- Algazi V.R., Duda R.O., Thompson D.M., Avendano C. Proceedings of the IEEE Workshop on Applications of Signal Processing to Audio and Electroacoustics. Mohonk Mountain House; New Paltz, NY: 2001. The CIPIC HRTF database; pp. 99–102.
-
- Arbogast T.L., Mason C.R., Jr., G.K The effect of spatial separation on informational and energetic masking of speech. J. Acoust. Soc. Am. 2002;112:2086–2098. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

