A Putative Acetylation System in Vibrio cholerae Modulates Virulence in Arthropod Hosts
- PMID: 30143508
- PMCID: PMC6193395
- DOI: 10.1128/AEM.01113-18
A Putative Acetylation System in Vibrio cholerae Modulates Virulence in Arthropod Hosts
Abstract
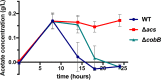
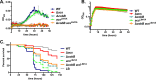
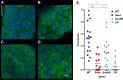
Acetylation is a broadly conserved mechanism of covalently modifying the proteome to precisely control protein activity. In bacteria, central metabolic enzymes and regulatory proteins, including those involved in virulence, can be targeted for acetylation. In this study, we directly link a putative acetylation system to metabolite-dependent virulence in the pathogen Vibrio cholerae We demonstrate that the cobB and yfiQ genes, which encode homologs of a deacetylase and an acetyltransferase, respectively, modulate V. cholerae metabolism of acetate, a bacterially derived short-chain fatty acid with important physiological roles in a diversity of host organisms. In Drosophila melanogaster, a model arthropod host for V. cholerae infection, the pathogen consumes acetate within the gastrointestinal tract, which contributes to fly mortality. We show that deletion of cobB impairs growth on acetate minimal medium, delays the consumption of acetate from rich medium, and reduces virulence of V. cholerae toward Drosophila These impacts can be reversed by complementing cobB or by introducing a deletion of yfiQ into the ΔcobB background. We further show that cobB controls the accumulation of triglycerides in the Drosophila midgut, which suggests that cobB directly modulates metabolite levels in vivo In Escherichia coli K-12, yfiQ is upregulated by cAMP-cAMP receptor protein (CRP), and we identified a similar pattern of regulation in V. cholerae, arguing that the system is activated in response to similar environmental cues. In summary, we demonstrate that proteins likely involved in acetylation can modulate the outcome of infection by regulating metabolite exchange between pathogens and their colonized hosts.IMPORTANCE The bacterium Vibrio cholerae causes severe disease in humans, and strains can persist in the environment in association with a wide diversity of host species. By investigating the molecular mechanisms that underlie these interactions, we can better understand constraints affecting the ecology and evolution of this global pathogen. The Drosophila model of Vibrio cholerae infection has revealed that bacterial regulation of acetate and other small metabolites from within the fly gastrointestinal tract is crucial for its virulence. Here, we demonstrate that genes that may modify the proteome of V. cholerae affect virulence toward Drosophila, most likely by modulating central metabolic pathways that control the consumption of acetate as well as other small molecules. These findings further highlight the many layers of regulation that tune bacterial metabolism to alter the trajectory of interactions between bacteria and their hosts.
Keywords: Drosophila melanogaster; Vibrio cholerae; acetate; acetyl-CoA; acetyl-CoA synthetase; acetylation; carbon metabolism; cholera; posttranslational modification.
Copyright © 2018 American Society for Microbiology.
Figures



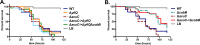


References
-
- Wang Q, Zhang Y, Yang C, Xiong H, Lin Y, Yao J, Li H, Xie L, Zhao W, Yao Y, Ning Z-B, Zeng R, Xiong Y, Guan K-L, Zhao S, Zhao G-P. 2010. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327:1004–1007. doi:10.1126/science.1179687. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

