Experimental Traumatic Brain Injury Identifies Distinct Early and Late Phase Axonal Conduction Deficits of White Matter Pathophysiology, and Reveals Intervening Recovery
- PMID: 30143572
- PMCID: PMC6181309
- DOI: 10.1523/JNEUROSCI.0819-18.2018
Experimental Traumatic Brain Injury Identifies Distinct Early and Late Phase Axonal Conduction Deficits of White Matter Pathophysiology, and Reveals Intervening Recovery
Abstract
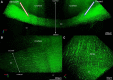
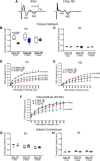
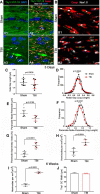
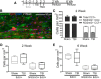
Traumatic brain injury (TBI) patients often exhibit slowed information processing speed that can underlie diverse symptoms. Processing speed depends on neural circuit function at synapses, in the soma, and along axons. Long axons in white matter (WM) tracts are particularly vulnerable to TBI. We hypothesized that disrupted axon-myelin interactions that slow or block action potential conduction in WM tracts may contribute to slowed processing speed after TBI. Concussive TBI in male/female mice was used to produce traumatic axonal injury in the corpus callosum (CC), similar to WM pathology in human TBI cases. Compound action potential velocity was slowed along myelinated axons at 3 d after TBI with partial recovery by 2 weeks, suggesting early demyelination followed by remyelination. Ultrastructurally, dispersed demyelinated axons and disorganized myelin attachment to axons at paranodes were apparent within CC regions exhibiting traumatic axonal injury. Action potential conduction is exquisitely sensitive to paranode abnormalities. Molecular identification of paranodes and nodes of Ranvier detected asymmetrical paranode pairs and abnormal heminodes after TBI. Fluorescent labeling of oligodendrocyte progenitors in NG2CreER;mTmG mice showed increased synthesis of new membranes extended along axons to paranodes, indicating remyelination after TBI. At later times after TBI, an overall loss of conducting axons was observed at 6 weeks followed by CC atrophy at 8 weeks. These studies identify a progression of both myelinated axon conduction deficits and axon-myelin pathology in the CC, implicating WM injury in impaired information processing at early and late phases after TBI. Furthermore, the intervening recovery reveals a potential therapeutic window.SIGNIFICANCE STATEMENT Traumatic brain injury (TBI) is a major global health concern. Across the spectrum of TBI severities, impaired information processing can contribute to diverse functional deficits that underlie persistent symptoms. We used experimental TBI to exploit technical advantages in mice while modeling traumatic axonal injury in white matter tracts, which is a key pathological feature of human TBI. A combination of approaches revealed slowed and failed signal conduction along with damage to the structure and molecular composition of myelinated axons in the white matter after TBI. An early regenerative response was not sustained yet reveals a potential time window for intervention. These insights into white matter abnormalities underlying axon conduction deficits can inform strategies to improve treatment options for TBI patients.
Keywords: CLARITY; axon damage; myelin; nerve conduction; node of Ranvier; paranode.
Copyright © 2018 Marion et al.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
