Immunomodulatory role of Keratin 76 in oral and gastric cancer
- PMID: 30143634
- PMCID: PMC6109110
- DOI: 10.1038/s41467-018-05872-4
Immunomodulatory role of Keratin 76 in oral and gastric cancer
Abstract
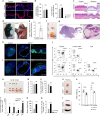
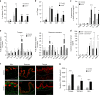
Keratin 76 (Krt76) is expressed in the differentiated epithelial layers of skin, oral cavity and squamous stomach. Krt76 downregulation in human oral squamous cell carcinomas (OSCC) correlates with poor prognosis. We show that genetic ablation of Krt76 in mice leads to spleen and lymph node enlargement, an increase in regulatory T cells (Tregs) and high levels of pro-inflammatory cytokines. Krt76-/- Tregs have increased suppressive ability correlated with increased CD39 and CD73 expression, while their effector T cells are less proliferative than controls. Loss of Krt76 increases carcinogen-induced tumours in tongue and squamous stomach. Carcinogenesis is further increased when Treg levels are elevated experimentally. The carcinogenesis response includes upregulation of pro-inflammatory cytokines and enhanced accumulation of Tregs in the tumour microenvironment. Tregs also accumulate in human OSCC exhibiting Krt76 loss. Our study highlights the role of epithelial cells in modulating carcinogenesis via communication with cells of the immune system.
Conflict of interest statement
F.M.W. is currently on secondment as Executive Chair of the UK Medical Research Council. The remaining authors declare no competing interests.
Figures






Comment in
-
The role of keratins in modulating carcinogenesis via communication with cells of the immune system.Cell Stress. 2019 Mar 23;3(4):136-138. doi: 10.15698/cst2019.04.184. Cell Stress. 2019. PMID: 31225508 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

