Mutant KLF1 in Adult Anemic Nan Mice Leads to Profound Transcriptome Changes and Disordered Erythropoiesis
- PMID: 30143664
- PMCID: PMC6109071
- DOI: 10.1038/s41598-018-30839-2
Mutant KLF1 in Adult Anemic Nan Mice Leads to Profound Transcriptome Changes and Disordered Erythropoiesis
Abstract
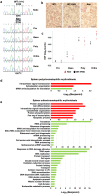
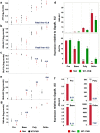
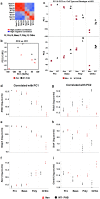
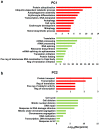
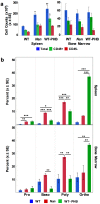
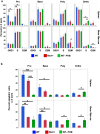
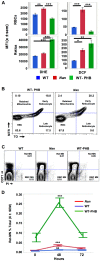
Anemic Nan mice carry a mutation (E339D) in the second zinc finger of erythroid transcription factor KLF1. Nan-KLF1 fails to bind a subset of normal KLF1 targets and ectopically binds a large set of genes not normally engaged by KLF1, resulting in a corrupted fetal liver transcriptome. Here, we performed RNAseq using flow cytometric-sorted spleen erythroid precursors from adult Nan and WT littermates rendered anemic by phlebotomy to identify global transcriptome changes specific to the Nan Klf1 mutation as opposed to anemia generally. Mutant Nan-KLF1 leads to extensive and progressive transcriptome corruption in adult spleen erythroid precursors such that stress erythropoiesis is severely compromised. Terminal erythroid differentiation is defective in the bone marrow as well. Principle component analysis reveals two major patterns of differential gene expression predicting that defects in basic cellular processes including translation, cell cycle, and DNA repair could contribute to disordered erythropoiesis and anemia in Nan. Significant erythroid precursor stage specific changes were identified in some of these processes in Nan. Remarkably, however, despite expression changes in large numbers of associated genes, most basic cellular processes were intact in Nan indicating that developing red cells display significant physiological resiliency and establish new homeostatic set points in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The mouse KLF1 Nan variant impairs nuclear condensation and erythroid maturation.PLoS One. 2019 Mar 28;14(3):e0208659. doi: 10.1371/journal.pone.0208659. eCollection 2019. PLoS One. 2019. PMID: 30921348 Free PMC article.
-
Novel roles for KLF1 in erythropoiesis revealed by mRNA-seq.Genome Res. 2012 Dec;22(12):2385-98. doi: 10.1101/gr.135707.111. Epub 2012 Jul 26. Genome Res. 2012. PMID: 22835905 Free PMC article.
-
Hemoglobin switching in mice carrying the Klf1Nan variant.Haematologica. 2021 Feb 1;106(2):464-473. doi: 10.3324/haematol.2019.239830. Haematologica. 2021. PMID: 32467144 Free PMC article.
-
KLF1 directly coordinates almost all aspects of terminal erythroid differentiation.IUBMB Life. 2010 Dec;62(12):886-90. doi: 10.1002/iub.404. IUBMB Life. 2010. PMID: 21190291 Review.
-
Severe anemia caused by dominant mutations in Krüppel-like factor 1 (KLF1).Mutat Res Rev Mutat Res. 2020 Oct-Dec;786:108336. doi: 10.1016/j.mrrev.2020.108336. Epub 2020 Oct 9. Mutat Res Rev Mutat Res. 2020. PMID: 33339573 Free PMC article. Review.
Cited by
-
Differential effects of RASA3 mutations on hematopoiesis are profoundly influenced by genetic background and molecular variant.PLoS Genet. 2020 Dec 28;16(12):e1008857. doi: 10.1371/journal.pgen.1008857. eCollection 2020 Dec. PLoS Genet. 2020. PMID: 33370780 Free PMC article.
-
Corrupted DNA-binding specificity and ectopic transcription underpin dominant neomorphic mutations in KLF/SP transcription factors.BMC Genomics. 2019 May 24;20(1):417. doi: 10.1186/s12864-019-5805-z. BMC Genomics. 2019. PMID: 31126231 Free PMC article.
-
EKLF/Klf1 regulates erythroid transcription by its pioneering activity and selective control of RNA Pol II pause-release.Cell Rep. 2022 Dec 20;41(12):111830. doi: 10.1016/j.celrep.2022.111830. Cell Rep. 2022. PMID: 36543143 Free PMC article.
-
Mutations in linker-2 of KLF1 impair expression of membrane transporters and cytoskeletal proteins causing hemolysis.Nat Commun. 2024 Aug 15;15(1):7019. doi: 10.1038/s41467-024-50579-4. Nat Commun. 2024. PMID: 39147774 Free PMC article.
-
The mouse KLF1 Nan variant impairs nuclear condensation and erythroid maturation.PLoS One. 2019 Mar 28;14(3):e0208659. doi: 10.1371/journal.pone.0208659. eCollection 2019. PLoS One. 2019. PMID: 30921348 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

