Estriol-mediated neuroprotection in multiple sclerosis localized by voxel-based morphometry
- PMID: 30144306
- PMCID: PMC6160650
- DOI: 10.1002/brb3.1086
Estriol-mediated neuroprotection in multiple sclerosis localized by voxel-based morphometry
Abstract
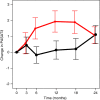
Introduction: Progressive gray matter (GM) atrophy is a hallmark of multiple sclerosis (MS). Cognitive impairment has been observed in 40%-70% of MS patients and has been linked to GM atrophy. In a phase 2 trial of estriol treatment in women with relapsing-remitting MS (RRMS), higher estriol levels correlated with greater improvement on the paced auditory serial addition test (PASAT) and imaging revealed sparing of localized GM in estriol-treated compared to placebo-treated patients. To better understand the significance of this GM sparing, the current study explored the relationships between the GM sparing and traditional MRI measures and clinical outcomes.
Methods: Sixty-two estriol- and forty-nine placebo-treated RRMS patients underwent clinical evaluations and brain MRI. Voxel-based morphometry (VBM) was used to evaluate voxelwise GM sparing from high-resolution T1-weighted scans.
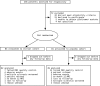
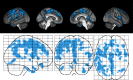
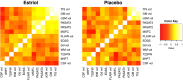
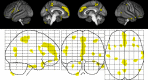
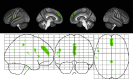
Results: A region of treatment-induced sparing (TIS) was defined as the areas where GM was spared in estriol- as compared to placebo-treated groups, localized primarily within the frontal and parietal cortices. We observed that TIS volume was directly correlated with improvement on the PASAT. Next, a longitudinal cognitive disability-specific atlas (DSA) was defined by correlating voxelwise GM volumes with PASAT scores, that is, areas where less GM correlated with less improvement in PASAT scores. Finally, overlap between the TIS and the longitudinal cognitive DSA revealed a specific region of cortical GM that was preserved in estriol-treated subjects that was associated with better performance on the PASAT.
Conclusions: Discovery of this region of overlap was biology driven, not based on an a priori structure of interest. It included the medial frontal cortex, an area previously implicated in problem solving and attention. These findings indicate that localized GM sparing during estriol treatment was associated with improvement in cognitive testing, suggesting a clinically relevant, disability-specific biomarker for clinical trials of candidate neuroprotective treatments in MS.
Keywords: atrophy; brain; estriol; magnetic resonance imaging; multiple sclerosis; voxel-based morphometry.
© 2018 The Authors. Brain and Behavior published by Wiley Periodicals, Inc.
Figures
Similar articles
-
Disability-Specific Atlases of Gray Matter Loss in Relapsing-Remitting Multiple Sclerosis.JAMA Neurol. 2016 Aug 1;73(8):944-53. doi: 10.1001/jamaneurol.2016.0966. JAMA Neurol. 2016. PMID: 27294295 Free PMC article. Clinical Trial.
-
Imaging patterns of gray and white matter abnormalities associated with PASAT and SDMT performance in relapsing-remitting multiple sclerosis.Mult Scler. 2019 Feb;25(2):204-216. doi: 10.1177/1352458517743091. Epub 2017 Nov 27. Mult Scler. 2019. PMID: 29173009
-
Detection and quantification of regional cortical gray matter damage in multiple sclerosis utilizing gradient echo MRI.Neuroimage Clin. 2015 Aug 18;9:164-75. doi: 10.1016/j.nicl.2015.08.003. eCollection 2015. Neuroimage Clin. 2015. PMID: 27330979 Free PMC article.
-
Gray Matter Atrophy in the Cortico-Striatal-Thalamic Network and Sensorimotor Network in Relapsing-Remitting and Primary Progressive Multiple Sclerosis.Neuropsychol Rev. 2021 Dec;31(4):703-720. doi: 10.1007/s11065-021-09479-3. Epub 2021 Feb 13. Neuropsychol Rev. 2021. PMID: 33582965 Review.
-
Urgent challenges in quantification and interpretation of brain grey matter atrophy in individual MS patients using MRI.Neuroimage Clin. 2018 Apr 26;19:466-475. doi: 10.1016/j.nicl.2018.04.023. eCollection 2018. Neuroimage Clin. 2018. PMID: 29984155 Free PMC article. Review.
Cited by
-
The effect of sex on multiple sclerosis risk and disease progression.Mult Scler. 2020 Apr;26(5):554-560. doi: 10.1177/1352458519892491. Epub 2020 Jan 22. Mult Scler. 2020. PMID: 31965884 Free PMC article. Review.
-
Pregnancy Is Associated with Impaired Transcription of Human Endogenous Retroviruses and of TRIM28 and SETDB1, Particularly in Mothers Affected by Multiple Sclerosis.Viruses. 2023 Mar 9;15(3):710. doi: 10.3390/v15030710. Viruses. 2023. PMID: 36992419 Free PMC article.
-
Gender disparities in cognitive impairment across neurological autoimmune disorders: a systematic review.Front Neurol. 2025 Apr 23;16:1555407. doi: 10.3389/fneur.2025.1555407. eCollection 2025. Front Neurol. 2025. PMID: 40337166 Free PMC article.
-
Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review.Biomedicines. 2022 Dec 1;10(12):3107. doi: 10.3390/biomedicines10123107. Biomedicines. 2022. PMID: 36551863 Free PMC article. Review.
-
All women with multiple sclerosis should start hormone replacement therapy at menopause unless contraindicated: Yes.Mult Scler. 2024 Aug;30(9):1107-1109. doi: 10.1177/13524585241255002. Epub 2024 Jun 22. Mult Scler. 2024. PMID: 38907632 Free PMC article. No abstract available.
References
-
- Battaglini, M. , Giorgio, A. , Stromillo, M. L. , Bartolozzi, M. L. , Guidi, L. , Federico, A. , & De Stefano, N. (2009). Voxel‐wise assessment of progression of regional brain atrophy in relapsing‐remitting multiple sclerosis. Journal of the Neurological Sciences, 282, 55–60. 10.1016/j.jns.2009.02.322 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

