Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells
- PMID: 30144404
- PMCID: PMC6248344
- DOI: 10.1016/j.bcp.2018.08.032
Glutaminase-1 stimulates the proliferation, migration, and survival of human endothelial cells
Abstract
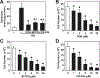
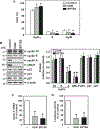
Glutaminase-1 (GLS1) is a mitochondrial enzyme found in endothelial cells (ECs) that metabolizes glutamine to glutamate and ammonia. Although glutaminolysis modulates the function of human umbilical vein ECs, it is not known whether these findings extend to human ECs beyond the fetal circulation. Furthermore, the molecular mechanism by which GLS1 regulates EC function is not defined. In this study, we show that the absence of glutamine in the culture media or the inhibition of GLS1 activity or expression blocked the proliferation and migration of ECs derived from the human umbilical vein, the human aorta, and the human microvasculature. GLS1 inhibition arrested ECs in the G0/G1 phase of the cell cycle and this was associated with a significant decline in cyclin A expression. Restoration of cyclin A expression via adenoviral-mediated gene transfer improved the proliferative, but not the migratory, response of GLS1-inhibited ECs. Glutamine deprivation or GLS1 inhibition also stimulated the production of reactive oxygen species and this was associated with a marked decline in heme oxygenase-1 (HO-1) expression. GLS1 inhibition also sensitized ECs to the cytotoxic effect of hydrogen peroxide and this was prevented by the overexpression of HO-1. In conclusion, the metabolism of glutamine by GLS1 promotes human EC proliferation, migration, and survival irrespective of the vascular source. While cyclin A contributes to the proliferative action of GLS1, HO-1 mediates its pro-survival effect. These results identify GLS1 as a promising therapeutic target in treating diseases associated with aberrant EC proliferation, migration, and viability.
Keywords: 6-Diazo-5-oxo-L-norleucine (DON, PubChem CID: 5359375); CB-839 (PubChem CID: 71577426); Endothelial cells; Glutaminase; Migration; Proliferation; bis-2-(5-Phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES, PubChem CID: 3372016).
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures



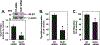

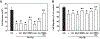

Similar articles
-
Glutamine metabolism via glutaminase 1 in autosomal-dominant polycystic kidney disease.Nephrol Dial Transplant. 2018 Aug 1;33(8):1343-1353. doi: 10.1093/ndt/gfx349. Nephrol Dial Transplant. 2018. PMID: 29420817 Free PMC article.
-
Deciphering the landscape of allosteric glutaminase 1 inhibitors as anticancer agents.Bioorg Chem. 2025 Jul 1;161:108523. doi: 10.1016/j.bioorg.2025.108523. Epub 2025 Apr 26. Bioorg Chem. 2025. PMID: 40311238 Review.
-
Glutaminase 1 regulates the release of extracellular vesicles during neuroinflammation through key metabolic intermediate alpha-ketoglutarate.J Neuroinflammation. 2018 Mar 14;15(1):79. doi: 10.1186/s12974-018-1120-x. J Neuroinflammation. 2018. PMID: 29540215 Free PMC article.
-
Inhibiting glutaminase in acute myeloid leukemia: metabolic dependency of selected AML subtypes.Oncotarget. 2016 Nov 29;7(48):79722-79735. doi: 10.18632/oncotarget.12944. Oncotarget. 2016. PMID: 27806325 Free PMC article.
-
Targeting GLS1 to cancer therapy through glutamine metabolism.Clin Transl Oncol. 2021 Nov;23(11):2253-2268. doi: 10.1007/s12094-021-02645-2. Epub 2021 May 23. Clin Transl Oncol. 2021. PMID: 34023970 Review.
Cited by
-
Altered Glutaminase 1 Activity During Neurulation and Its Potential Implications in Neural Tube Defects.Front Pharmacol. 2020 Jun 19;11:900. doi: 10.3389/fphar.2020.00900. eCollection 2020. Front Pharmacol. 2020. PMID: 32636743 Free PMC article.
-
Exploring the relationship between anastasis and mitochondrial ROS-mediated ferroptosis in metastatic chemoresistant cancers: a call for investigation.Front Immunol. 2024 Jul 2;15:1428920. doi: 10.3389/fimmu.2024.1428920. eCollection 2024. Front Immunol. 2024. PMID: 39015566 Free PMC article. Review.
-
Dual inhibition of glutaminase and carnitine palmitoyltransferase decreases growth and migration of glutaminase inhibition-resistant triple-negative breast cancer cells.J Biol Chem. 2019 Jun 14;294(24):9342-9357. doi: 10.1074/jbc.RA119.008180. Epub 2019 Apr 30. J Biol Chem. 2019. PMID: 31040181 Free PMC article.
-
The influence of endothelial metabolic reprogramming on the tumor microenvironment.Oncogene. 2025 Jan;44(2):51-63. doi: 10.1038/s41388-024-03228-5. Epub 2024 Nov 20. Oncogene. 2025. PMID: 39567756 Free PMC article. Review.
-
Endothelial senescence in vascular diseases: current understanding and future opportunities in senotherapeutics.Exp Mol Med. 2023 Jan;55(1):1-12. doi: 10.1038/s12276-022-00906-w. Epub 2023 Jan 4. Exp Mol Med. 2023. PMID: 36599934 Free PMC article. Review.
References
-
- Lacey JM, Wilmore DW. Is glutamine a conditionally essential amino acid? Nutr Rev 48:297–309, 1990. - PubMed
-
- Curi R, Newholme P, Procopio J, Langranha C, Gorjao R, Pithon-Curi TC. Glutamine, gene expression and cell function. Front Biosci 2007;12:344–357. - PubMed
-
- Young VR, Ajami AM. Glutamine: the emperor or his clothes. J Nutr 2001;131:2449S2459S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

