Dexmedetomidine is effective and safe during NIV in infants and young children with acute respiratory failure
- PMID: 30144795
- PMCID: PMC6109351
- DOI: 10.1186/s12887-018-1256-y
Dexmedetomidine is effective and safe during NIV in infants and young children with acute respiratory failure
Abstract
Background: Noninvasive ventilation (NIV) is increasingly utilized in infants and young children, though associated with high failure rates due to agitation and poor compliance, mostly if patient-ventilator synchronization is required.
Methods: A retrospective cohort study was carried out in an academic pediatric intensive care unit (PICU). Dexmedetomidine (DEX) was infused as unique sedative in 40 consecutive pediatric patients (median age 16 months) previously showing intolerance and agitation during NIV application.
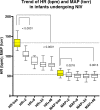
Results: During NIV clinical application both COMFORT-B Score and Richmond Agitation-Sedation Scale (RASS) were serially evaluated. Four patients experiencing NIV failure, all due to pulmonary condition worsening, required intubation and invasive ventilation. 36 patients were successfully weaned from NIV under DEX sedation and discharged from PICU. All patients survived until home discharge.
Conclusion: Our data suggest that DEX may represent an effective sedative agent in infants and children showing agitation during NIV. Early use of DEX in infants/children receiving NIV for acute respiratory failure (ARF) should be considered safe and capable of improving NIV, thus permitting both lung recruitment and patient-ventilator synchronization.
Keywords: Acute respiratory failure; Dexmedetomidine; Infant; Non invasive ventilation; Sedation.
Conflict of interest statement
Ethics approval and consent to participate
The Ethical Committee “Comitato Etico dell’Università Cattolica Del Sacro Cuore” approved the study and waived the need for informed consent. Reference number 26257/14.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Yañez LJ, Yunge M, Emilfork M, Lapadula M, Alcántara A, Fernández C, Lozano J, Contreras M, Conto L, Arevalo C, Gayan A, Hernández F, Pedraza M, Feddersen M, Bejares M, Morales M, Mallea F, Glasinovic M, Cavada G. A prospective, randomized, controlled trial of noninvasive ventilation in pediatric acute respiratory failure. Pediatr Crit Care Med. 2008;9(5):484–489. doi: 10.1097/PCC.0b013e318184989f. - DOI - PubMed
-
- Essouri S, Carroll C. and Group., Pediatric Acute Lung Injury Consensus Conference. Noninvasive support and ventilation for pediatric acute respiratory distress syndrome: proceedings from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;6(5 Suppl 1):S102–S110. doi: 10.1097/PCC.0000000000000437. - DOI - PubMed
-
- Pancera CF, Hayashi M, Fregnani JH, Negri EM, Deheinzelin D, de Camargo B. Noninvasive ventilation in immunocompromised pediatric patients: eight years of experience in a pediatric oncology intensive care unit. J Pediatr Hematol Oncol. 2008;30(7):533–538. doi: 10.1097/MPH.0b013e3181754198. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

