Too sweet: Problems of protein glycation in the eye
- PMID: 30145354
- PMCID: PMC8351608
- DOI: 10.1016/j.exer.2018.08.017
Too sweet: Problems of protein glycation in the eye
Abstract
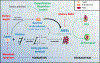
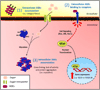
Laboratory and epidemiological data indicate that high blood sugar levels and/or consuming high glycemia diets are linked to multiple age-related diseases, including age-related macular degeneration, cataract, Parkinson's disease, Alzheimer's disease, diabetic retinopathy, and, apparently glaucoma. High concentrations of blood sugar and perturbations of the systems that regulate blood sugar lead to the accumulation of advanced-glycation end products (AGEs). AGEs are toxic compounds that are formed from the combination of sugars and their metabolites with biomolecules in a non-enzymatic biochemical reaction called glycation. In vitro and in vivo data indicate that high sugar consumption is associated with accumulation of AGEs in a variety of human tissues. Hyperglycemia, along with an oxidative environment and limited cell proliferation in many ocular tissues, encourages formation and precludes dilution of AGEs and associated damage by cell division. These circumstances make many eye tissues vulnerable to glycation-derived damage. Here, we summarize research regarding glycation-induced ocular tissue dysfunction and its contribution to the onset and development of eye disorders. We also discuss how management of carbohydrate nutrition may provide a low-cost way to ameliorate the progression of AGEs-related diseases, including age related macular degeneration and some cataracts, as they do for cardiovascular disease and diabetes.
Keywords: Advanced glycation-end products; Age-related macular degeneration; Aging; Cataracts; Cornea; Diabetes; Diabetic retinopathy; Glycemic index; Optic nerve; Retina; Trabecular meshwork; Vitreous.
Copyright © 2018. Published by Elsevier Ltd.
Figures
References
-
- Abiko T, Abiko A, Ishiko S, Takeda M, Horiuchi S, Yoshida A, 1999. Relationship between autofluorescence and advanced glycation end products in diabetic lenses. Exp. Eye Res. 68, 361–366. - PubMed
-
- Ahmed MU, Thorpe SR, Baynes JW, 1986. Identification of N-Carboxymethyllysine as a degradation product of fructoselysine in glycatedprotein. J. Biol. Chem. 261, 4889–4894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

