Short-term effects of low-concentration atropine eye drops on pupil size and accommodation in young adult subjects
- PMID: 30145612
- PMCID: PMC6208716
- DOI: 10.1007/s00417-018-4112-8
Short-term effects of low-concentration atropine eye drops on pupil size and accommodation in young adult subjects
Abstract
Purpose: A single eye drop containing 0.01% atropine every evening has previously been found to inhibit myopia progression in young adults. We have tested the short-term effects of very low-dose atropine eye drops on pupil sizes and accommodation in young adult subjects.
Methods: Fourteen eyes of young adult subjects participated in the clinical observation. A single eye drop was applied with concentrations of either 0.01%, 0.005%, or 0.001% in the evening. Baseline parameters were measured before atropine application. Changes of pupil sizes, under photopic and mesopic conditions, as well as accommodation amplitudes were observed over the next day and analyzed by paired the Wilcoxon signed-rank test.
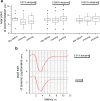
Results: The pupil was significantly dilated 12 h after instillation of 0.01% atropine eye drops, both under photopic (3.3 ± 0.5 mm vs. 4.9 ± 0.9 mm) and mesopic (4.8 ± 0.7 mm vs. 6.1 ± 0.7 mm) conditions. Pupil sizes recovered over the day but were still significantly larger in the evening, compared to the baseline parameters measured on the day before (3.9 ± 0.5 mm vs. 5.3 ± 0.6 mm). The subjective near point of accommodation was reduced from 8.0 ± 2.4 to 6.6 ± 2.8 dpt in the morning and to 7.0 ± 2.9 dpt in the evening. At 0.005%, the pattern of results remained still similar, although the magnitude of the effects was generally smaller. At 0.001%, pupil sizes were still weakly significantly larger in the morning.
Conclusions: At a dose of 0.01%, clinically significant short-term effects were detected on pupil size and accommodation for at least 24 h. At the lowest dose of 0.001%, only tiny effects on pupil size were detectable.
Keywords: Accommodation; Atropine; Eye drops; Myopia; Pupil size.
Conflict of interest statement
Conflict of interest
Author A Fricke has received a speaker honorarium of DGII in 2016.
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest other than the speaker honorarium (A Fricke) (such as honoraria; educational grants; participation in speaker bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements) or non-financial interest (such as personal or professional relationship, affiliations, knowledge, or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Figures



Similar articles
-
Dose Dependent Effects of Atropine on Static and Dynamic Pupil and Accommodation Metrics in Young Adults.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):34. doi: 10.1167/iovs.66.6.34. Invest Ophthalmol Vis Sci. 2025. PMID: 40492989 Free PMC article. Clinical Trial.
-
Effect of low-dose atropine on myopia progression, pupil diameter and accommodative amplitude: low-dose atropine and myopia progression.Br J Ophthalmol. 2020 Nov;104(11):1535-1541. doi: 10.1136/bjophthalmol-2019-315440. Epub 2020 Feb 21. Br J Ophthalmol. 2020. PMID: 32086237 Clinical Trial.
-
Low-Concentration Atropine for Myopia Progression (LAMP) Study: A Randomized, Double-Blinded, Placebo-Controlled Trial of 0.05%, 0.025%, and 0.01% Atropine Eye Drops in Myopia Control.Ophthalmology. 2019 Jan;126(1):113-124. doi: 10.1016/j.ophtha.2018.05.029. Epub 2018 Jul 6. Ophthalmology. 2019. PMID: 30514630 Clinical Trial.
-
Efficacy and Safety of 8 Atropine Concentrations for Myopia Control in Children: A Network Meta-Analysis.Ophthalmology. 2022 Mar;129(3):322-333. doi: 10.1016/j.ophtha.2021.10.016. Epub 2021 Oct 22. Ophthalmology. 2022. PMID: 34688698 Review.
-
Topical Atropine in the Control of Myopia.Asia Pac J Ophthalmol (Phila). 2016 Nov/Dec;5(6):424-428. doi: 10.1097/APO.0000000000000232. Asia Pac J Ophthalmol (Phila). 2016. PMID: 27898446 Review.
Cited by
-
The Impact of Clinical Atropine Use in Taiwanese Schoolchildren: Changes in Physiological Characteristics and Visual Functions.Children (Basel). 2021 Nov 15;8(11):1054. doi: 10.3390/children8111054. Children (Basel). 2021. PMID: 34828767 Free PMC article.
-
The ascending arousal system promotes optimal performance through mesoscale network integration in a visuospatial attentional task.Netw Neurosci. 2021 Nov 30;5(4):890-910. doi: 10.1162/netn_a_00205. eCollection 2021. Netw Neurosci. 2021. PMID: 35024535 Free PMC article.
-
Evaluation of Static and Dynamic Pupil and Light Sensitivity to a Single Drop of Various Concentrations of Low-Dose Atropine (0.01%, 0.025%, and 0.05%).Life (Basel). 2025 Feb 11;15(2):278. doi: 10.3390/life15020278. Life (Basel). 2025. PMID: 40003687 Free PMC article.
-
Effect of 0.025% atropine on ocular biometry changes during accommodation.Ophthalmic Physiol Opt. 2025 May;45(3):865-876. doi: 10.1111/opo.13485. Epub 2025 Mar 7. Ophthalmic Physiol Opt. 2025. PMID: 40052515 Free PMC article.
-
Changes in accommodation and vergence parameters with topical use of 0.025% and 0.05% atropine in myopes aged between 7 and 17 years.Eye (Lond). 2025 Jul 19. doi: 10.1038/s41433-025-03908-w. Online ahead of print. Eye (Lond). 2025. PMID: 40684055
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

