Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination
- PMID: 30146391
- PMCID: PMC6152889
- DOI: 10.1016/j.chom.2018.08.001
Alveolar Macrophages Provide an Early Mycobacterium tuberculosis Niche and Initiate Dissemination
Abstract
Mycobacterium tuberculosis (Mtb) infection is initiated in the distal airways, but the bacteria ultimately disseminate to the lung interstitium. Although various cell types, including alveolar macrophages (AM), neutrophils, and permissive monocytes, are known to be infected with Mtb, the initially infected cells as well as those that mediate dissemination from the alveoli to the lung interstitium are unknown. In this study, using a murine infection model, we reveal that early, productive Mtb infection occurs almost exclusively within airway-resident AM. Thereafter Mtb-infected, but not uninfected, AM localize to the lung interstitium through mechanisms requiring an intact Mtb ESX-1 secretion system. Relocalization of infected AM precedes Mtb uptake by recruited monocyte-derived macrophages and neutrophils. This dissemination process is driven by non-hematopoietic host MyD88/interleukin-1 receptor inflammasome signaling. Thus, interleukin-1-mediated crosstalk between Mtb-infected AM and non-hematopoietic cells promotes pulmonary Mtb infection by enabling infected cells to disseminate from the alveoli to the lung interstitium.
Keywords: ESX-1; IL-1; alveolar macrophages; granuloma; innate immunity; lung; pulmonary tuberculosis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
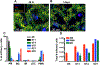
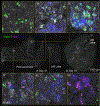
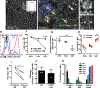
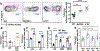
Comment in
-
Mycobacterium tuberculosis Joyrides Alveolar Macrophages into the Pulmonary Interstitium.Cell Host Microbe. 2018 Sep 12;24(3):331-333. doi: 10.1016/j.chom.2018.08.011. Cell Host Microbe. 2018. PMID: 30212645
References
-
- Bermudez LE, Sangari FJ, Kolonoski P, Petrofsky M, and Goodman J (2002). The efficiency of the translocation of Mycobacterium tuberculosis across a bilayer of epithelial and endothelial cells as a model of the alveolar wall is a consequence of transport within mononuclear phagocytes and invasion of alveolar epithelial cells. Infect Immun 70, 140–146. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

