Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes
- PMID: 30146667
- PMCID: PMC6110307
- DOI: 10.1186/s40169-018-0204-7
Viral antigens detectable in CSF exosomes from patients with retrovirus associated neurologic disease: functional role of exosomes
Abstract
Background: HTLV-1 infects over 20 million people worldwide and causes a progressive neuroinflammatory disorder in a subset of infected individuals called HTLV-1 associated myelopathy/tropical spastic paraparesis (HAM/TSP). The detection of HTLV-1 specific T cells in the cerebrospinal fluid (CSF) suggests this disease is immunopathologically mediated and that it may be driven by viral antigens. Exosomes are microvesicles originating from the endosomal compartment that are shed into the extracellular space by various cell types. It is now understood that several viruses take advantage of this mode of intercellular communication for packaging of viral components as well. We sought to understand if this is the case in HTLV-1 infection, and specifically if HTLV-1 proteins can be found in the CSF of HAM/TSP patients where we know free virus is absent, and furthermore, if exosomes containing HTLV-1 Tax have functional consequences.
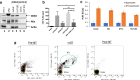
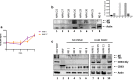
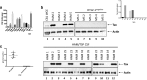
Results: Exosomes that were positive for HTLV-1 Tax by Western blot were isolated from HAM/TSP patient PBMCs (25/36) in ex vivo cultures by trapping exosomes from culture supernatants. HTLV-1 seronegative PBMCs did not have exosomes with Tax (0/12), (Fisher exact test, p = 0.0001). We were able to observe HAM/TSP patient CSF (12/20) containing Tax+ exosomes but not in HTLV-1 seronegative MS donors (0/5), despite the absence of viral detection in the CSF supernatant (Fisher exact test p = 0.0391). Furthermore, exosomes cultivated from HAM/TSP PBMCs were capable of sensitizing target cells for HTLV-1 specific CTL lysis.
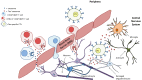
Conclusion: Cumulatively, these results show that there are HTLV-1 proteins present in exosomes found in virus-free CSF. HAM/TSP PBMCs, particularly CD4+CD25+ T cells, can excrete these exosomes containing HTLV-1 Tax and may be a source of the exosomes found in patient CSF. Importantly, these exosomes are capable of sensitizing an HTLV-1 specific immune response, suggesting that they may play a role in the immunopathology observed in HAM/TSP. Given the infiltration of HTLV-1 Tax-specific CTLs into the CNS of HAM/TSP patients, it is likely that exosomes may also contribute to the continuous activation and inflammation observed in HAM/TSP, and may suggest future targeted therapies in this disorder.
Keywords: Cell-free virus; Exosomes; HTLV-1 Tax; Nanotraps; Specific lysis; Spontaneous proliferation.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
