Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue
- PMID: 30146913
- PMCID: PMC6393219
- DOI: 10.1080/21691401.2018.1499660
Comparison of covalently and physically cross-linked collagen hydrogels on mediating vascular network formation for engineering adipose tissue
Abstract
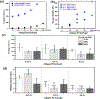
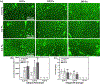
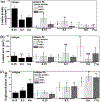
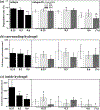
Timely tissue vascularization and integration of engineered tissues into a patient plays an important role in the successful translation of engineered tissues into clinically relevant therapies. To decrease the time needed to vascularize an engineered adipose tissue, suitable local microenvironments provided by hydrogels to support cell-based functional vascular network formation have been investigated. Using the same biomolecule in solution, two types of hydrogels can be obtained: a "physical hydrogel" which is thermal-induced self-assemble fibril initiation and growth, due to amino and carboxyl telopeptides on collagen chains, and a "chemical hydrogel" which results from the covalently cross-linking of the side chains induced by one step enzyme mediation in aqueous solution. In this paper, we compare the capability of engineering vascular network and large-sized vascularized adipose tissue in vivo in different types of collagen hydrogels, physical and chemical crosslinking. The relationships between vascular network formation and hydrogel properties for the two types of hydrogels are discussed. Finally, we successfully engineered a vascularized adipose tissue construct (∼877.6 adipocytes/mm2; 94% area of a construct) in the absence of exogenous cytokines in chemical covalently crosslinking cell-laden hydrogel. These results show manipulating the polymerized methods of a hydrogel could not only modulate vascular network formation, but also regenerate adipose tissue in vivo.
Keywords: Adipose tissue engineering vascular network; endothelial colony forming cells; hydrogel.
Figures






References
-
- Smith P, Adams WP, Lipschitz AH, et al. Autologous human fat grafting: Effect of harvesting and preparation techniques on adipocyte graft survival [Article; Proceedings Paper]. Plast Reconstr Surg. 2006. May;117(6):1836–1844. doi: 10.1097/01.prs.0000218825.77014.78. PubMed PMID: ; English. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
