Matrix metalloproteinase 2/9-triggered-release micelles for inhaled drug delivery to treat lung cancer: preparation and in vitro/in vivo studies
- PMID: 30147314
- PMCID: PMC6095127
- DOI: 10.2147/IJN.S166584
Matrix metalloproteinase 2/9-triggered-release micelles for inhaled drug delivery to treat lung cancer: preparation and in vitro/in vivo studies
Abstract
Background: Improvement in drug accumulation in the lungs through inhalation administration and high expression of MMP2 and MMP9 in lung tumors have both been widely reported.
Methods: MMP2/9-triggered-release micelles were constructed and in vitro and in vivo studies of inhalation administration against lung tumor carried out. Pluronic P123 (P123) was modified with GPLGIAGQ-NH2 (GQ8) peptide to obtain P123-GQ8 (PG). MMP2/9-triggered-release micelles were constructed using PG and succinylated gelatin (SG) and loading paclitaxel (Ptx). To study biodistribution of micelles, DiR encapsulated in micelles was dosed to rats via intravenous injection or inhalation before ex vivo imaging for detecting DiR quantity in lungs. And B16F10 lung cancer-bearing nude mice were chosen as animal models to evaluate in vivo efficacy of MMP2/9-triggered-release micelles.
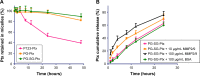
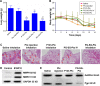
Results: Ptx-release efficiency from PG-SG-Ptx micelles was MMP2/9-concentration-dependent. For A549 cells, PG-SG-Ptx cytotoxicity was significantly greater (P<0.001) compared to P123-Ptx. Aerosol inhalation was chosen as the method of administration. In biodistribution experiment, DiR quantity in lungs was 5.8%±0.4% of that in major organs, while the ratio was 38.8%±0.5% for inhalation. For B16F10 lung cancer-bearing nude mice, the efficacy of inhalation of PG-SG-Ptx was significantly higher (P<0.001) than Taxol inhalation and injected PG-SG-Ptx. Inhaled PG-SG-Ptx also significantly inhibited the expression of Pgp in lung cancer.
Conclusion: Inhalation of MMP2/9-triggered-release micelles increased tumor sensitivity to chemotherapeutics and reduced the toxicity of chemotherapy to healthy lung cells, which has great potential in lung cancer therapy.
Keywords: P-glycoprotein; Pluronic; inhalation; matrix metalloproteinases; paclitaxel.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures






References
-
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. - PubMed
-
- Ettinger D, Wood D, Aisner D, et al. Non-small cell lung cancer, version 5.2017: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. - PubMed
-
- Spiro SG, Porter JC. Lung cancer: where are we today? Current advances in staging and nonsurgical treatment. Am J Respir Crit Care Med. 2002;166(9):1166–1196. - PubMed
-
- Ahmad A, Gadgeel S, editors. Lung Cancer and Personalized Medicine: Current Knowledge and Therapies. Heidelberg: Springer; 2016.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

