(Poly)cationic λ3-Iodane Mediated Oxidative Ring Expansion of Secondary Alcohols
- PMID: 30147437
- PMCID: PMC6107298
- DOI: 10.1002/ejoc.201800118
(Poly)cationic λ3-Iodane Mediated Oxidative Ring Expansion of Secondary Alcohols
Abstract
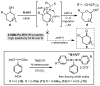
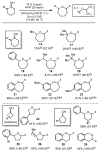
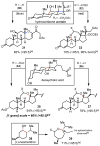
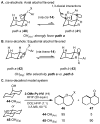
Herein, we report a simplified approach to the synthesis of medium-ring ethers through the electrophilic activation of secondary alcohols with (poly)cationic λ3-iodanes (N-HVI). Excellent levels of selectivity are achieved for C-O bond migration over established α-elimination pathways, enabled by the unique reactivity of a novel 2-OMe-pyridine-ligated N-HVI. The resulting HFIP-acetals are readily derivatized with a range of nucleophiles, providing a versatile functional handle for subsequent manipulations. The utility of this methodology for late-stage natural product derivatization was also demonstrated, providing a new tool for diversity-oriented synthesis and complexity-to-diversity (CTD) efforts. Preliminary mechanistic investigations reveal a strong effect of alcohol conformation on reactive pathway, thus providing a predictive power in the application of this approach to complex molecule synthesis.
Keywords: cyclic ethers; hypervalent iodine; medium-ring; ring expansion.
Figures


References
-
- Illuminati G, Mandolini L. Acc Chem Res. 1981;14:95.
-
- Molander GA. Acc Chem Res. 1998
- Hoberg JO. Tetrahedron. 1998;54:12631.
- Kleinke AS, Webb D, Jamison TF. Tetrahedron. 2012;68:6999.
-
- Lovering F, Bikker J, Humblet C. J Med Chem. 2009;52:6752. - PubMed
-
- Lovering F. Med Chem Commun. 2013;4:515.
-
- Huigens RW, III, Morrison KC, Hicklin RW, Flood TA, Jr, Richter MF, Hergenrother PJ. Nat Chem. 2013;5:195. - PMC - PubMed
- Kopp F, Stratton CF, Akella LB, Tan DS. Nat Chem Biol. 2012;8:358. - PMC - PubMed
- Kumar N, Kiuchi M, Tallarico AJA, Schreiber SL. Org Lett. 2005 - PubMed
- Shimokawa J. Tetrahedron Lett. 2014;55:6156.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
