Transcriptional Profiling Suggests Extensive Metabolic Rewiring of Human and Mouse Macrophages during Early Interferon Alpha Responses
- PMID: 30147442
- PMCID: PMC6083555
- DOI: 10.1155/2018/5906819
Transcriptional Profiling Suggests Extensive Metabolic Rewiring of Human and Mouse Macrophages during Early Interferon Alpha Responses
Abstract
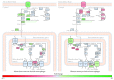
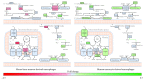
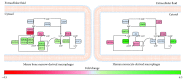
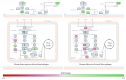
Emerging evidence suggests that cellular metabolism plays a critical role in regulating immune activation. Alterations in energy and lipid and amino acid metabolism have been shown to contribute to type I interferon (IFN) responses in macrophages, but the relationship between metabolic reprogramming and the establishment of early antiviral function remains poorly defined. Here, we used transcriptional profiling datasets to develop global metabolic signatures associated with early IFN-α responses in two primary macrophage model systems: mouse bone marrow-derived macrophages (BMM) and human monocyte-derived macrophages (MDM). Short-term stimulation with IFN-α (<4 hours) was associated with significant metabolic rewiring, with >500 metabolic genes altered in mouse and human macrophage models. Pathway and network analysis identified alterations in genes associated with cellular bioenergetics, cellular oxidant status, cAMP/AMP and cGMP/GMP ratios, branched chain amino acid catabolism, cell membrane composition, fatty acid synthesis, and β-oxidation as key features of early IFN-α responses. These changes may have important implications for initial establishment of antiviral function in these cells.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

