C. elegans MANF Homolog Is Necessary for the Protection of Dopaminergic Neurons and ER Unfolded Protein Response
- PMID: 30147641
- PMCID: PMC6095968
- DOI: 10.3389/fnins.2018.00544
C. elegans MANF Homolog Is Necessary for the Protection of Dopaminergic Neurons and ER Unfolded Protein Response
Abstract
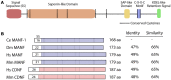
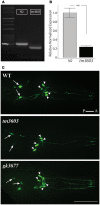
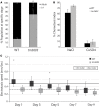
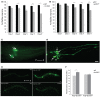
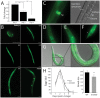
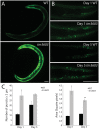
Neurotrophic factors (NTFs) are important for the development, function, and survival of neurons in the mammalian system. Mesencephalic astrocyte-derived neurotrophic factor (MANF) and cerebral dopamine neurotrophic factor (CDNF) are two recently identified members of a novel family of NTFs in vertebrates that function to protect dopaminergic neurons. Although these genes are conserved across eukaryotes, their mechanism of neuroprotection is not fully understood. Sequence searches for MANF/CDNF homologs in invertebrates have identified a single ortholog that is most related to MANF. Here we report the in vivo characterization of the MANF gene, manf-1, in the nematode Caenorhabditis elegans. We found that manf-1 mutants have an accelerated, age-dependent decline in the survival of dopaminergic neurons. The animals also show increased endoplasmic reticulum (ER) stress, as revealed by reporter gene expression analysis of hsp-4, an ER chaperone BiP/GRP78 homolog, suggesting that a failure to regulate the ER unfolded protein response (ER-UPR) may be a contributing factor to dopaminergic neurodegeneration. Expression studies of manf-1 revealed that the gene is broadly expressed in a pattern that matches closely with hsp-4. Consistent with the requirements of manf-1 in the ER-UPR, we found that aggregates of α-Synuclein, a major constituent of Lewy bodies, were significantly increased in body wall muscles of manf-1 mutant animals. Overall, our work demonstrates the important role of manf-1 in dopaminergic neuronal survival and the maintenance of ER homeostasis in C. elegans.
Keywords: C. elegans; CDNF; ER stress; MANF; Parkinson's disease; dopamine; manf-1; neurodegeneration.
Figures
References
-
- Bargmann C. I. (2006). Chemosensation in C. elegans, in The, C. elegans Research Community, ed WormBook. (WormBook; ), 1–29. Available online at: http://www.wormbook.org - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

