Allergic Asthma Favors Brucella Growth in the Lungs of Infected Mice
- PMID: 30147700
- PMCID: PMC6095999
- DOI: 10.3389/fimmu.2018.01856
Allergic Asthma Favors Brucella Growth in the Lungs of Infected Mice
Abstract
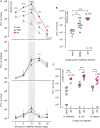
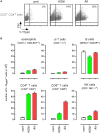
Allergic asthma is a chronic Th2 inflammatory disease of the lower airways affecting a growing number of people worldwide. The impact of infections and microbiota composition on allergic asthma has been investigated frequently. Until now, however, there have been few attempts to investigate the impact of asthma on the control of infectious microorganisms and the underlying mechanisms. In this work, we characterize the consequences of allergic asthma on intranasal (i.n.) infection by Brucella bacteria in mice. We observed that i.n. sensitization with extracts of the house dust mite Dermatophagoides farinae or the mold Alternaria alternata (Alt) significantly increased the number of Brucella melitensis, Brucella suis, and Brucella abortus in the lungs of infected mice. Microscopic analysis showed dense aggregates of infected cells composed mainly of alveolar macrophages (CD11c+ F4/80+ MHCII+) surrounded by neutrophils (Ly-6G+). Asthma-induced Brucella susceptibility appears to be dependent on CD4+ T cells, the IL-4/STAT6 signaling pathway and IL-10, and is maintained in IL-12- and IFN-γR-deficient mice. The effects of the Alt sensitization protocol were also tested on Streptococcus pneumoniae and Mycobacterium tuberculosis pulmonary infections. Surprisingly, we observed that Alt sensitization strongly increases the survival of S. pneumoniae infected mice by a T cell and STAT6 independent signaling pathway. In contrast, the course of M. tuberculosis infection is not affected in the lungs of sensitized mice. Our work demonstrates that the impact of the same allergic sensitization protocol can be neutral, negative, or positive with regard to the resistance of mice to bacterial infection, depending on the bacterial species.
Keywords: Brucella melitensis; Mycobacterium tuberculosis; Streptococcus pneumoniae; allergic asthma; brucellosis; infection.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

