Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol
- PMID: 30147712
- PMCID: PMC6104845
- DOI: 10.5194/acp-17-2103-2017
Nitrate radicals and biogenic volatile organic compounds: oxidation, mechanisms, and organic aerosol
Abstract
Oxidation of biogenic volatile organic compounds (BVOC) by the nitrate radical (NO3) represents one of the important interactions between anthropogenic emissions related to combustion and natural emissions from the biosphere. This interaction has been recognized for more than 3 decades, during which time a large body of research has emerged from laboratory, field, and modeling studies. NO3-BVOC reactions influence air quality, climate and visibility through regional and global budgets for reactive nitrogen (particularly organic nitrates), ozone, and organic aerosol. Despite its long history of research and the significance of this topic in atmospheric chemistry, a number of important uncertainties remain. These include an incomplete understanding of the rates, mechanisms, and organic aerosol yields for NO3-BVOC reactions, lack of constraints on the role of heterogeneous oxidative processes associated with the NO3 radical, the difficulty of characterizing the spatial distributions of BVOC and NO3 within the poorly mixed nocturnal atmosphere, and the challenge of constructing appropriate boundary layer schemes and non-photochemical mechanisms for use in state-of-the-art chemical transport and chemistry-climate models. This review is the result of a workshop of the same title held at the Georgia Institute of Technology in June 2015. The first half of the review summarizes the current literature on NO3-BVOC chemistry, with a particular focus on recent advances in instrumentation and models, and in organic nitrate and secondary organic aerosol (SOA) formation chemistry. Building on this current understanding, the second half of the review outlines impacts of NO3-BVOC chemistry on air quality and climate, and suggests critical research needs to better constrain this interaction to improve the predictive capabilities of atmospheric models.
Conflict of interest statement
Competing interests. The authors declare that they have no conflict of interest.
Figures

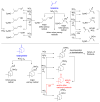
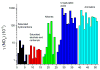


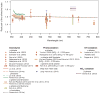
References
-
- Abida O, Mielke LH, Osthoff HD. Observation of gas-phase peroxynitrous and peroxynitric acid during the photolysis of nitrate in acidified frozen solutions. Chem Phys Lett. 2011;511:187–192. doi: 10.1016/j.cplett.2011.06.055. - DOI
-
- Alessandrini S, Ferrero E. A hybrid Lagrangian–Eulerian particle model for reacting pollutant dispersion in non-homogeneous non-isotropic turbulence. Physica A. 2009;388:1375–1387. doi: 10.1016/j.physa.2008.12.015. - DOI
-
- Aliwell SR, Jones RL. Measurement of atmospheric NO3 2. Diurnal variation of stratospheric NO3 at midlatitude. Geophys Res Lett. 1996a;23:2589–2592.
-
- Aliwell SR, Jones RL. Measurement of atmospheric NO3 1. Improved removal of water vapour absorption features in the analysis for NO3. Geophys Res Lett. 1996b;23:2585–2588.
-
- Aliwell SR, Jones RL. Measurements of tropospheric NO3 at midlatitude. J Geophys Res. 1998;103:5719–5727.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
