GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues
- PMID: 30148676
- PMCID: PMC6355063
- DOI: 10.1096/fj.201800171R
GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues
Abstract
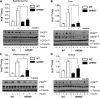
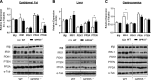
Emerging evidence indicates that G-protein coupled receptor 55 (GPR55), a nonclassic receptor of the endocannabinoid system that is activated by L-α-lysophosphatidylinositol and various cannabinoid ligands, may regulate endocrine function and energy metabolism. We examined how GPR55 deficiency and modulation affects insulin signaling in skeletal muscle, adipose tissue, and liver alongside expression analysis of proteins implicated in insulin action and energy metabolism. We show that GPR55-null mice display decreased insulin sensitivity in these tissues, as evidenced by reduced phosphorylation of PKB/Akt and its downstream targets, concomitant with increased adiposity and reduced physical activity relative to wild-type counterparts. Impaired tissue insulin sensitivity coincided with reduced insulin receptor substrate-1 abundance in skeletal muscle, whereas in liver and epididymal fat it was associated with increased expression of the 3-phosphoinoistide lipid phosphatase, phosphatase and tensin homolog. In contrast, GPR55 activation enhanced insulin signaling in cultured skeletal muscle cells, adipocytes, and hepatocytes; this response was negated by receptor antagonists and GPR55 gene silencing in L6 myotubes. Sustained GPR55 antagonism in 3T3-L1 adipocytes enhanced expression of proteins implicated in lipogenesis and promoted triglyceride accumulation. Our findings identify GPR55 as a positive regulator of insulin action and adipogenesis and as a potential therapeutic target for countering obesity-induced metabolic dysfunction and insulin resistance.-Lipina, C., Walsh, S. K., Mitchell, S. E., Speakman, J. R., Wainwright, C. L., Hundal, H. S. GPR55 deficiency is associated with increased adiposity and impaired insulin signaling in peripheral metabolic tissues.
Keywords: adipogenesis; cannabinoid receptor; endocannabinoid; liver; skeletal muscle.
Conflict of interest statement
This work was supported by the Biotechnology and Biological Sciences Research Council (BBSRC) and Diabetes UK. The authors declare no conflicts of interest.
Figures








References
-
- Osei-Hyiaman D., DePetrillo M., Pacher P., Liu J., Radaeva S., Bátkai S., Harvey-White J., Mackie K., Offertáler L., Wang L., Kunos G. (2005) Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J. Clin. Invest. 115, 1298–1305 - PMC - PubMed
-
- Matias I., Gonthier M. P., Orlando P., Martiadis V., De Petrocellis L., Cervino C., Petrosino S., Hoareau L., Festy F., Pasquali R., Roche R., Maj M., Pagotto U., Monteleone P., Di Marzo V. (2006) Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J. Clin. Endocrinol. Metab. 91, 3171–3180 - PubMed
-
- Côté M., Matias I., Lemieux I., Petrosino S., Alméras N., Després J. P., Di Marzo V. (2007) Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int. J. Obes. 31, 692–699 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

