Modelling mesenchymal stromal cell growth in a packed bed bioreactor with a gas permeable wall
- PMID: 30148832
- PMCID: PMC6110476
- DOI: 10.1371/journal.pone.0202079
Modelling mesenchymal stromal cell growth in a packed bed bioreactor with a gas permeable wall
Abstract
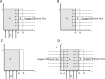
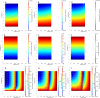
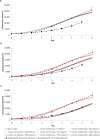
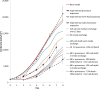
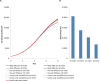
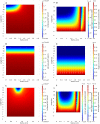
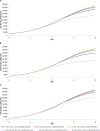
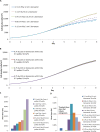
A mathematical model was developed for mesenchymal stromal cell (MSC) growth in a packed bed bioreactor that improves oxygen availability by allowing oxygen diffusion through a gas-permeable wall. The governing equations for oxygen, glucose and lactate, the inhibitory waste product, were developed assuming Michaelis-Menten kinetics, together with an equation for the medium flow based on Darcy's Law. The conservation law for the cells includes the effects of inhibition as the cells reach confluence, nutrient and waste product concentrations, and the assumption that the cells can migrate on the scaffold. The equations were solved using the finite element package, COMSOL. Previous experimental results collected using a packed bed bioreactor with gas permeable walls to expand MSCs produced a lower cell yield than was obtained using a traditional cell culture flask. This mathematical model suggests that the main contributors to the observed low cell yield were a non-uniform initial cell seeding profile and a potential lag phase as cells recovered from the initial seeding procedure. Lactate build-up was predicted to have only a small effect at lower flow rates. Thus, the most important parameters to optimise cell expansion in the proliferation of MSCs in a bioreactor with gas permeable wall are the initial cell seeding protocol and the handling of the cells during the seeding process. The mathematical model was then used to identify and characterise potential enhancements to the bioreactor design, including incorporating a central gas permeable capillary to further enhance oxygen availability to the cells. Finally, to evaluate the issues and limitations that might be encountered scale-up of the bioreactor, the mathematical model was used to investigate modifications to the bioreactor design geometry and packing density.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Weber C, Freimark D, Portner R, Pino-Grace P, Pohl S, Wallrapp C, et al. Expansion of human mesenchymal stem cells in a fixed-bed bioreactor system based on non-porous glass carrier—part A: inoculation, cultivation, and cell harvest procedures. The International journal of artificial organs. 2010;33(8):512–25. Epub 2010/09/28. . - PubMed
-
- De Napoli IE, Scaglione S, Giannoni P, Quarto R, Catapano G. Mesenchymal stem cell culture in convection-enhanced hollow fibre membrane bioreactors for bone tissue engineering. Journal of Membrane Science. 2011;379(1–2):341–52. 10.1016/j.memsci.2011.06.001. - DOI
-
- Li M, Tilles AW, Milwid JM, Hammad M, Lee J, Yarmush ML, et al. Phenotypic and functional characterization of human bone marrow stromal cells in hollow-fibre bioreactors. Journal of tissue engineering and regenerative medicine. 2012;6(5):369–77. Epub 2011/06/29. 10.1002/term.439 ; PubMed Central PMCID: PMCPMC3183117. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

