A high-throughput screen of real-time ATP levels in individual cells reveals mechanisms of energy failure
- PMID: 30148842
- PMCID: PMC6110572
- DOI: 10.1371/journal.pbio.2004624
A high-throughput screen of real-time ATP levels in individual cells reveals mechanisms of energy failure
Abstract
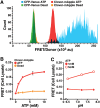
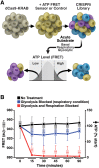
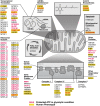
Insufficient or dysregulated energy metabolism may underlie diverse inherited and degenerative diseases, cancer, and even aging itself. ATP is the central energy carrier in cells, but critical pathways for regulating ATP levels are not systematically understood. We combined a pooled clustered regularly interspaced short palindromic repeats interference (CRISPRi) library enriched for mitochondrial genes, a fluorescent biosensor, and fluorescence-activated cell sorting (FACS) in a high-throughput genetic screen to assay ATP concentrations in live human cells. We identified genes not known to be involved in energy metabolism. Most mitochondrial ribosomal proteins are essential in maintaining ATP levels under respiratory conditions, and impaired respiration predicts poor growth. We also identified genes for which coenzyme Q10 (CoQ10) supplementation rescued ATP deficits caused by knockdown. These included CoQ10 biosynthetic genes associated with human disease and a subset of genes not linked to CoQ10 biosynthesis, indicating that increasing CoQ10 can preserve ATP in specific genetic contexts. This screening paradigm reveals mechanisms of metabolic control and genetic defects responsive to energy-based therapies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Comment in
-
Dissecting the regulation and function of ATP at the single-cell level.PLoS Biol. 2018 Dec 14;16(12):e3000095. doi: 10.1371/journal.pbio.3000095. eCollection 2018 Dec. PLoS Biol. 2018. PMID: 30550559 Free PMC article.
References
-
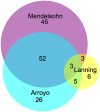
- Lanning NJ, Looyenga BD, Kauffman AL, Niemi NM, Sudderth J, Deberardinis RJ, et al. A Mitochondrial RNAi Screen Defines Cellular Bioenergetic Determinants and Identifies an Adenylate Kinase as a Key Regulator of ATP Levels. Cell Rep. 2014;7(3):907–17. 10.1016/j.celrep.2014.03.065 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 CA168370/CA/NCI NIH HHS/United States
- U54 CA196519/CA/NCI NIH HHS/United States
- R00 CA204602/CA/NCI NIH HHS/United States
- T32 GM007618/GM/NIGMS NIH HHS/United States
- R01 DA036858/DA/NIDA NIH HHS/United States
- K12 HD000850/HD/NICHD NIH HHS/United States
- S10 RR028962/RR/NCRR NIH HHS/United States
- P30 NS069496/NS/NINDS NIH HHS/United States
- DP2 GM119139/GM/NIGMS NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- T32 GM008568/GM/NIGMS NIH HHS/United States
- C06 RR018928/RR/NCRR NIH HHS/United States
- K99 CA204602/CA/NCI NIH HHS/United States
- R01 NS091902/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

