Comparison of antidiabetic drugs added to sulfonylurea monotherapy in patients with type 2 diabetes mellitus: A network meta-analysis
- PMID: 30148851
- PMCID: PMC6110472
- DOI: 10.1371/journal.pone.0202563
Comparison of antidiabetic drugs added to sulfonylurea monotherapy in patients with type 2 diabetes mellitus: A network meta-analysis
Abstract
Aims: This study aimed to investigate the efficacy and safety of dual therapy comprising sulfonylurea (SU) plus antidiabetic drugs for the treatment of type 2 diabetes mellitus (T2DM).
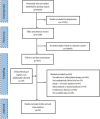
Methods: We searched the PubMed, Cochrane library, and Embase databases for randomized clinical trials (≥24 weeks) published up to December 28, 2017. Subsequently, we conducted pairwise and network meta-analyses to calculate the odds ratios (ORs) and mean differences (MDs) with 95% confidence intervals (CIs) of the outcomes.
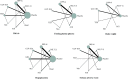
Results: The final analyses included 24 trials with a total of 10,032 patients. Compared with placebo, all treatment regimens were associated with a significantly higher risk of hypoglycemia, except the combinations of SU plus sodium-glucose co-transporter-2 inhibitor (SGLT-2i) [OR, 1.35 (95% CI: 0.81 to 2.25)] or alpha-glucosidase inhibitor (AGI) [OR, 1.16 (95% CI: 0.55 to 2.44)]. Notably, the combination of SU plus glucagon-like peptide-1 receptor agonist (GLP-1RA) was associated with the most significant increase in the risk of hypoglycemia. Furthermore, all SU-based combination regimens reduced the glycated hemoglobin (HbA1c) and fasting plasma glucose levels (FPG). However, only combinations containing SGLT-2i [MD, -1.00 kg (95% CI: -1.73 to -0.27)] and GLP-1RA [MD, -0.56 kg (95% CI: -1.10 to -0.02)] led to weight loss.
Conclusions: Our findings highlight the importance of considering the risk of hypoglycemia when selecting antidiabetic drugs to be administered concomitantly with SU. Although all classes of antidiabetic drugs improved glucose control when administered in combination with SU, SGLT-2i might be the best option with respect to factors such as hypoglycemia and body weight.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Efficacy and safety of vildagliptin in patients with type 2 diabetes mellitus inadequately controlled with dual combination of metformin and sulphonylurea.Diabetes Obes Metab. 2014 May;16(5):403-9. doi: 10.1111/dom.12229. Epub 2013 Dec 2. Diabetes Obes Metab. 2014. PMID: 24199686 Free PMC article. Clinical Trial.
-
Efficacy and safety of dipeptidyl peptidase-4 inhibitors and metformin as initial combination therapy and as monotherapy in patients with type 2 diabetes mellitus: a meta-analysis.Diabetes Obes Metab. 2014 Jan;16(1):30-7. doi: 10.1111/dom.12174. Epub 2013 Jul 16. Diabetes Obes Metab. 2014. PMID: 23803146
-
Comparison of GLP-1 Receptor Agonists, SGLT-2 Inhibitors, and DPP-4 Inhibitors as an Add-On Drug to Insulin Combined With Oral Hypoglycemic Drugs: Umbrella Review.J Diabetes Res. 2024 Jul 20;2024:8145388. doi: 10.1155/2024/8145388. eCollection 2024. J Diabetes Res. 2024. PMID: 39072050 Free PMC article.
-
Glycemic efficacy and safety of glucagon-like peptide-1 receptor agonist on top of sodium-glucose co-transporter-2 inhibitor treatment compared to sodium-glucose co-transporter-2 inhibitor alone: A systematic review and meta-analysis of randomized controlled trials.Diabetes Res Clin Pract. 2019 Dec;158:107927. doi: 10.1016/j.diabres.2019.107927. Epub 2019 Nov 13. Diabetes Res Clin Pract. 2019. PMID: 31733280
-
Canagliflozin in Conjunction With Sulfonylurea Maintains Glycemic Control and Weight Loss Over 52 Weeks: A Randomized, Controlled Trial in Patients With Type 2 Diabetes Mellitus.Clin Ther. 2017 Nov;39(11):2230-2242.e2. doi: 10.1016/j.clinthera.2017.10.003. Epub 2017 Nov 3. Clin Ther. 2017. PMID: 29103664 Clinical Trial.
Cited by
-
Effects of different hypoglycaemic drugs on beta-cell function in type 2 diabetes mellitus: a systematic review and network meta-analysis.Eur J Med Res. 2025 Feb 21;30(1):121. doi: 10.1186/s40001-025-02368-y. Eur J Med Res. 2025. PMID: 39985051 Free PMC article.
-
Comparative analysis of the efficacies of probiotic supplementation and glucose-lowering drugs for the treatment of type 2 diabetes: A systematic review and meta-analysis.Front Nutr. 2022 Jul 18;9:825897. doi: 10.3389/fnut.2022.825897. eCollection 2022. Front Nutr. 2022. PMID: 35923194 Free PMC article.
-
Efficacy and Safety of Ertugliflozin in Patients With Diabetes Mellitus Inadequately Controlled by Sulfonylurea Monotherapy: a Substudy of VERTIS CV.Diabetes Ther. 2021 Apr;12(4):1175-1192. doi: 10.1007/s13300-021-01018-w. Epub 2021 Mar 10. Diabetes Ther. 2021. PMID: 33694093 Free PMC article.
-
Prevalence and predictors of clinical inertia in patients with type 2 diabetes who were treated with a single oral antidiabetic drug.J Diabetes Investig. 2023 Jan;14(1):81-91. doi: 10.1111/jdi.13923. Epub 2022 Oct 13. J Diabetes Investig. 2023. PMID: 36229998 Free PMC article.
-
Prescription of Sulphonylureas among Patients with Type 2 Diabetes Mellitus in Italy: Results from the Retrospective, Observational Multicentre Cross-Sectional SUSCIPE (Sulphonyl_UreaS_Correct_Internal_Prescription_Evaluation) Study.Diabetes Ther. 2020 Sep;11(9):2105-2119. doi: 10.1007/s13300-020-00871-5. Epub 2020 Jul 30. Diabetes Ther. 2020. PMID: 32734558 Free PMC article.
References
-
- IDF DIABETES ATLAS– 8TH EDITION. Available from http://www.diabetesatlas.org/across-the-globe.html. Accessed 28 Dec 2017.
-
- Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140–9. 10.2337/dc14-2441 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

