Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction
- PMID: 30149090
- PMCID: PMC6239905
- DOI: 10.1016/j.plipres.2018.08.002
Properties, metabolism and roles of sulfogalactosylglycerolipid in male reproduction
Abstract
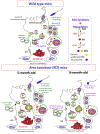
Sulfogalactosylglycerolipid (SGG, aka seminolipid) is selectively synthesized in high amounts in mammalian testicular germ cells (TGCs). SGG is an ordered lipid and directly involved in cell adhesion. SGG is indispensable for spermatogenesis, a process that greatly depends on interaction between Sertoli cells and TGCs. Spermatogenesis is disrupted in mice null for Cgt and Cst, encoding two enzymes essential for SGG biosynthesis. Sperm surface SGG also plays roles in fertilization. All of these results indicate the significance of SGG in male reproduction. SGG homeostasis is also important in male fertility. Approximately 50% of TGCs become apoptotic and phagocytosed by Sertoli cells. SGG in apoptotic remnants needs to be degraded by Sertoli lysosomal enzymes to the lipid backbone. Failure in this event leads to a lysosomal storage disorder and sub-functionality of Sertoli cells, including their support for TGC development, and consequently subfertility. Significantly, both biosynthesis and degradation pathways of the galactosylsulfate head group of SGG are the same as those of sulfogalactosylceramide (SGC), a structurally related sulfoglycolipid important for brain functions. If subfertility in males with gene mutations in SGG/SGC metabolism pathways manifests prior to neurological disorder, sperm SGG levels might be used as a reporting/predicting index of the neurological status.
Keywords: Lipid rafts; Lipidomics; Male fertility; Male reproduction; Mass spectrometry; Seminolipid; Sulfogalactosylglycerolipid.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflicts of interest
None
Figures








References
-
- Ishizuka I, Suzuki M, Yamakawa T, Isolation and characterization of a novel sulfoglycolipid, seminolipid, from boar testis and spermatozoa, J. Biochem 73 (1973) 77–87. - PubMed
-
- Tanphaichitr N, Bou Khalil M, Weerachatyanukul W, Kates M, Xu H, Carmona E, Attar M, Carrier D, Physiological and biophysical properties of male germ cell sulfogalactosylglycerolipid, in: De Vriese S (Ed.), Lipid Metabolism and Male Fertility, AOCS Press, Champaign, IL, 2003, pp. 125–148.
-
- Tanphaichitr N, Carmona E, Bou Khalil M, Xu H, Berger T, Gerton GL, New insights into sperm-zona pellucida interaction: involvement of sperm lipid rafts, Front. Biosci 12 (2007) 1748–1766. - PubMed
-
- Tanphaichitr N, Faull KF, Yaghoubian A, Xu H, Lipid rafts and sulfogalactosylglycerolipid (SGG) in sperm functions: consensus and controversy, Trends Glycosci. Glycotech 19(106) (2007) 67–83.
-
- Rajendran L, Simons K, Lipid rafts and membrane dynamics, J. Cell Sci 118(Pt 6) (2005) 1099–1102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

