Risk Factors for Parainfluenza Virus Lower Respiratory Tract Disease after Hematopoietic Cell Transplantation
- PMID: 30149147
- PMCID: PMC6310631
- DOI: 10.1016/j.bbmt.2018.08.021
Risk Factors for Parainfluenza Virus Lower Respiratory Tract Disease after Hematopoietic Cell Transplantation
Abstract
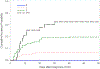
Parainfluenza virus (PIV) infection can progress from upper respiratory tract infection (URTI) to lower respiratory tract disease (LRTD) in immunocompromised hosts. Risk factors for progression to LRTD and presentation with LRTD without prior URTI are poorly defined. Hematopoietic cell transplant (HCT) recipients with PIV infection were retrospectively analyzed using standardized definitions of LRTD. PIV was detected in 540 HCT recipients; 343 had URTI alone and 197 (36%) had LRTD (possible, 76; probable, 19; proven, 102). Among 476 patients with positive nasopharyngeal samples, the cumulative incidence of progression to probable/proven LRTD by day 40 was 12%, with a median time to progression of 7 days (range, 2 to 40). In multivariable analysis monocytopenia (hazard ratio, 2.22; P = .011), steroid use ≥1mg/kg prior to diagnosis (hazard ratio, 1.89; P = .018), co-pathogen detection in blood (hazard ratio, 3.21; P = .027), and PIV type 3 (hazard ratio, 3.57; P = .032) were associated with increased progression risk. In the absence of all 4 risk factors no patients progressed to LRTD, whereas progression risk increased to >30% if 3 or more risk factors were present. Viral load or ribavirin use appeared to have no effect on progression. Among 121 patients with probable/proven LRTD, 64 (53%) presented LRTD without prior URTI, and decreased lung function before infection and lower respiratory co-pathogens were risk factors for this presentation. Mortality was unaffected by the absence of prior URTI. We conclude that the risk of progression to probable/proven LRTD exceeded 30% with ≥3 risk factors. To detect all cases of LRTD, virologic testing of lower respiratory samples is required regardless of URTI symptoms.
Keywords: Hematopoietic cell transplantation; Lower respiratory tract disease; Parainfluenza virus; Progression; Ribavirin.
Copyright © 2018. Published by Elsevier Inc.
Conflict of interest statement
Figures




References
-
- Fry AM, Curns AT, Harbour K, Hutwagner L, Holman RC, Anderson LJ. Seasonal trends of human parainfluenza viral infections: United States, 1990–2004. Clinical infectious diseases 2006; 43: 1016–22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

