Colocalization of Aluminum and Iron in Nuclei of Nerve Cells in Brains of Patients with Alzheimer's Disease
- PMID: 30149443
- PMCID: PMC6218123
- DOI: 10.3233/JAD-171108
Colocalization of Aluminum and Iron in Nuclei of Nerve Cells in Brains of Patients with Alzheimer's Disease
Abstract
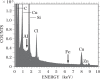
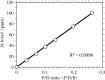
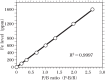
Increasing evidence indicates that metal-induced oxidative stress plays a pivotal role in the pathogenesis of Alzheimer's disease (AD). Recently, the presence of 8-hydroxydeoxyguanosine, a biomarker of oxidative DNA damage, was demonstrated in nuclear DNA (nDNA) in the AD brain. Iron (Fe) is a pro-oxidant metal capable of generating hydroxyl radicals that can oxidize DNA, and aluminum (Al) has been reported to facilitate Fe-mediated oxidation. In the present study, we examined the elements contained in the nuclei of nerve cells in AD brains using scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDS). Our results demonstrated that Al and Fe were colocalized in the nuclei of nerve cells in the AD brain. Within the nuclei, the highest levels of both Al and Fe were measured in the nucleolus. The SEM-EDS analysis also revealed the colocalization of Al and Fe in the heterochromatin and euchromatin in neuronal nuclei in the AD brain. Notably, the levels of Al and Fe in the nucleus of nerve cells in the AD brain were markedly higher than those in age-matched control brains. We hypothesize that the colocalization of Al and Fe in the nucleus of nerve cells might induce oxidative damage to nDNA and concurrently inhibit the repair of oxidatively damaged nDNA. An imbalance caused by the increase in DNA damage and the decrease in DNA repair activities might lead to the accumulation of unrepaired damaged DNA, eventually causing neurodegeneration and the development of AD.
Keywords: Aluminum; Alzheimer’s disease; DNA damage; Fenton reaction; electron microscopy; energy-dispersive X-ray spectroscopy; iron; neurodegeneration; oxidative stress.
Figures





References
-
- Smith MA, Rottkamp CA, Nunomura A, Raina AK, Perry G (2000) Oxidative stress in Alzheimer’s disease. Biochim Biophys Acta 1502, 139–144. - PubMed
-
- Kawahara M (2005) Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimers Dis 8, 171–182. - PubMed
-
- Walton JR (2014) Chronic aluminum intake causes Alzheimer’s disease: Applying Sir Austin Bradford Hill’s causality criteria. J Alzheimers Dis 40, 765–838. - PubMed
-
- Hebert LE, Scherr PA, Beckett LA, Albert MS, Pilgrim DM, Chown MJ, Funkenstein HH, Evans DA (1995) Age-specific incidence of Alzheimer’s disease in a community population. JAMA 273, 1354–1359. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

