Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis
- PMID: 30149516
- PMCID: PMC6162800
- DOI: 10.3390/cells7090116
Cytotoxic Constituents from the Sclerotia of Poria cocos against Human Lung Adenocarcinoma Cells by Inducing Mitochondrial Apoptosis
Abstract
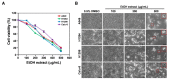
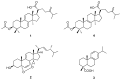
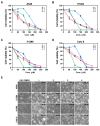
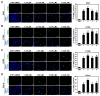
Previous studies have revealed the antitumor potential of Poria cocos Wolf against a broad spectrum of cancers. However, the biological activity of P. cocos against lung cancer, which is known as the leading cause of cancer mortality worldwide, and its underlying chemical and molecular basis, remain to be investigated. We aimed to evaluate the in vitro cytotoxicity of P. cocos toward human lung adenocarcinoma cells with different p53 statuses, to identify the bioactive constituents of P. cocos, and explicate the molecular mechanisms underlying the cytotoxicity of these constituents in human lung adenocarcinoma cells. An EtOH extract of the sclerotia of P. cocos exhibited cytotoxicity toward four human lung cancer cell lines: A549, H1264, H1299, and Calu-6, regardless of their p53 status. Chemical investigation of the extract resulted in the isolation of two triterpenoids, dehydroeburicoic acid monoacetate (1) and acetyl eburicoic acid (4); a sterol, 9,11-dehydroergosterol peroxide (2); and a diterpenoid, dehydroabietic acid (3). All of the isolated compounds were cytotoxic to the lung adenocarcinoma cell lines, exhibiting IC50 values ranging from 63.6 μM to 171.0 μM at 48 h of treatment. The cytotoxicity of the extract and the isolated compounds were found to be mediated by apoptosis, and accompanied by elevated Bax expression and/or Bcl-2 phosphorylation along with caspase-3 activation. Our data demonstrate that the sclerotium of P. cocos and its four bioactive constituents (1⁻4) exert cytotoxicity against human lung adenocarcinoma cells, regardless of their p53 status, by inducing apoptosis associated with mitochondrial perturbation, and proposing the potential to employ P. cocos in the treatment of lung cancer.
Keywords: Poria cocos; apoptosis; cytotoxicity; diterpenoid; lung cancer; polyporaceae; sterol; triterpenoid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Fitzmaurice C., Allen C., Barber R.M., Barregard L., Bhutta Z.A., Brenner H., Dicker D.J., Chimed-Orchir O., Dandona R., Dandona L., et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015: A systematic analysis for the global burden of disease study. JAMA Oncol. 2017;3:524–548. - PMC - PubMed
-
- Maehara Y., Tsujitani S., Saeki H., Oki E., Yoshinaga K., Emi Y., Morita M., Kohnoe S., Kakeji Y., Yano T., et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN®): Review of development and future perspectives. Surg. Today. 2012;42:8–28. doi: 10.1007/s00595-011-0075-7. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

