Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring
- PMID: 30149597
- PMCID: PMC6163729
- DOI: 10.3390/ijms19092514
Liquid Biopsy Biomarkers in Bladder Cancer: A Current Need for Patient Diagnosis and Monitoring
Abstract
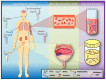
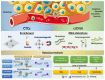
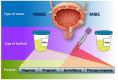
Bladder Cancer (BC) represents a clinical and social challenge due to its high incidence and recurrence rates, as well as the limited advances in effective disease management. Currently, a combination of cytology and cystoscopy is the routinely used methodology for diagnosis, prognosis and disease surveillance. However, both the poor sensitivity of cytology tests as well as the high invasiveness and big variation in tumour stage and grade interpretation using cystoscopy, emphasizes the urgent need for improvements in BC clinical guidance. Liquid biopsy represents a new non-invasive approach that has been extensively studied over the last decade and holds great promise. Even though its clinical use is still compromised, multiple studies have recently focused on the potential application of biomarkers in liquid biopsies for BC, including circulating tumour cells and DNA, RNAs, proteins and peptides, metabolites and extracellular vesicles. In this review, we summarize the present knowledge on the different types of biomarkers, their potential use in liquid biopsy and clinical applications in BC.
Keywords: biomarkers; bladder cancer; liquid biopsy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

