miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy
- PMID: 30149628
- PMCID: PMC6162422
- DOI: 10.3390/cancers10090289
miRNA-205 Nanoformulation Sensitizes Prostate Cancer Cells to Chemotherapy
Abstract
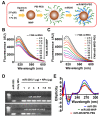
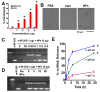
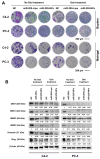
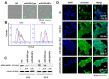
The therapeutic application of microRNA(s) in the field of cancer has generated significant attention in research. Previous studies have shown that miR-205 negatively regulates prostate cancer cell proliferation, metastasis, and drug resistance. However, the delivery of miR-205 is an unmet clinical need. Thus, the development of a viable nanoparticle platform to deliver miR-205 is highly sought. A novel magnetic nanoparticle (MNP)-based nanoplatform composed of an iron oxide core with poly(ethyleneimine)-poly(ethylene glycol) layer(s) was developed. An optimized nanoplatform composition was confirmed by examining the binding profiles of MNPs with miR-205 using agarose gel and fluorescence methods. The novel formulation was applied to prostate cancer cells for evaluating cellular uptake, miR-205 delivery, and anticancer, antimetastasis, and chemosensitization potentials against docetaxel treatment. The improved uptake and efficacy of formulations were studied with confocal imaging, flow cytometry, proliferation, clonogenicity, Western blot, q-RT-PCR, and chemosensitization assays. Our findings demonstrated that the miR-205 nanoplatform induces significant apoptosis and enhancing chemotherapeutic effects in prostate cancer cells. Overall, these study results provide a strong proof-of-concept for a novel nonviral-based nanoparticle protocol for effective microRNA delivery to prostate cancer cells.
Keywords: chemosensitivity and EMT; docetaxel; miR-205; prostate cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Corcoran C., Rani S., O’Brien K., O’Neill A., Prencipe M., Sheikh R., Webb G., McDermott R., Watson W., Crown J., et al. Docetaxel-resistance in prostate cancer: Evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PloS ONE. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. - DOI - PMC - PubMed
-
- Cengiz E., Karaca B., Kucukzeybek Y., Gorumlu G., Gul M.K., Erten C., Atmaca H., Uzunoglu S., Karabulut B., Sanli U.A., et al. Overcoming drug resistance in hormone- and drug-refractory prostate cancer cell line, pc-3 by docetaxel and gossypol combination. Mol. Biol. Rep. 2010;37:1269–1277. doi: 10.1007/s11033-009-9501-y. - DOI - PubMed
-
- Pekarik V., Gumulec J., Masarik M., Kizek R., Adam V. Prostate cancer, miRNAs, metallothioneins and resistance to cytostatic drugs. Curr. Med. Chem. 2013;20:534–544. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

