Chitosan⁻Carboxymethylcellulose-Based Polyelectrolyte Complexation and Microcapsule Shell Formulation
- PMID: 30149641
- PMCID: PMC6163483
- DOI: 10.3390/ijms19092521
Chitosan⁻Carboxymethylcellulose-Based Polyelectrolyte Complexation and Microcapsule Shell Formulation
Abstract
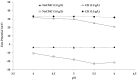
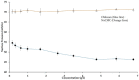
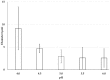
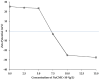
Chitosan (CH)⁻carboxymethyl cellulose sodium salt (NaCMC) microcapsules containing paraffin oil were synthesized by complex formation, and crosslinked with glutaraldehyde (GTA). The electrostatic deposition of NaCMC onto the CH-coated paraffin oil emulsion droplets was demonstrated by zeta potential and optical microscopy. The optimal process conditions were identified in terms of pH of the aqueous solution (5.5) and CH/NaCMC mass ratio (1:1). Encapsulation of paraffin oil and microcapsule morphology were analyzed by ATR-FTIR and SEM, respectively. The effect of GTA crosslinking on paraffin oil latent heat was investigated by DSC and combined with the values of encapsulation efficiency and core content, supporting the compact shell formation.
Keywords: carboxymethyl cellulose sodium salt; chitosan; emulsion; microcapsule; polyelectrolyte complex.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

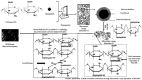
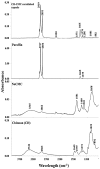

References
-
- Gärdlund L., Wågberg L., Gernandt R. Polyelectrolyte Complexes for Surface Modification of Wood Fibres: II. Influence of Complexes on Wet and Dry Strength of Paper. Colloids Surf. A Physicochem. Eng. Asp. 2003;218:137–149. doi: 10.1016/S0927-7757(02)00588-5. - DOI
-
- Schwarz H.H., Lukáš J., Richau K. Surface and Permeability Properties of Membranes from Polyelectrolyte Complexes and Polyelectrolyte Surfactant Complexes. J. Membr. Sci. 2003;218:1–9. doi: 10.1016/S0376-7388(02)00548-3. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

