The geography of sex: sexual conflict, environmental gradients and local loss of sex in facultatively parthenogenetic animals
- PMID: 30150220
- PMCID: PMC6125730
- DOI: 10.1098/rstb.2017.0422
The geography of sex: sexual conflict, environmental gradients and local loss of sex in facultatively parthenogenetic animals
Erratum in
-
Correction to 'The geography of sex: sexual conflict, environmental gradients and local loss of sex in facultatively parthenogenetic animals'.Philos Trans R Soc Lond B Biol Sci. 2019 Apr 1;374(1769):20190001. doi: 10.1098/rstb.2019.0001. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 30967093 Free PMC article. No abstract available.
Abstract
Obligately asexual organisms tend to occur at higher altitudes or latitudes and occupy larger ranges than their obligately sexual relatives-a phenomenon called geographical parthenogenesis. Some facultatively parthenogenetic organisms that reproduce both sexually and asexually also exhibit spatial variation in reproductive mode. Theory suggests that sexual conflict and mate limitation can determine the relative frequency of sex in facultative parthenogens, but the effect of these dynamics on spatial distributions is unknown. Here, we use individual-based models to investigate whether these dynamics can generate local differences in the reproductive mode in a facultatively parthenogenetic metapopulation occupying an environmental gradient. We find that selection for resistance and high fecundity creates positive epistasis in virgin females between a mutant allele for parthenogenesis and alleles for resistance, resulting in female-biased sex ratios and higher resistance and coercion towards the productive 'core' of the metapopulation. However, steeper environmental gradients, which lead to lower density and less mating at the 'edge', generate female bias without promoting coercion or resistance. Our analysis shows that local adaptation of facultatively parthenogenetic populations subject to sexual conflict and productivity gradients can generate striking spatial variation suggesting new patterns for empirical investigation. These findings could also help to explain the rarity of facultative parthenogenesis in animals.This article is part of the theme issue 'Linking local adaptation with the evolution of sex differences'.
Keywords: environmental gradient; facultative parthenogenesis; geographical parthenogenesis; individual-based model; paradox of sex; sexual conflict.
© 2018 The Authors.
Conflict of interest statement
We have no competing interests.
Figures

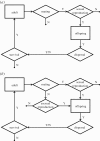
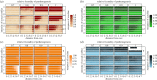

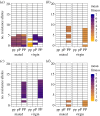
References
-
- Daly M. 1978. The cost of mating. Am. Nat. 112, 771–774. (10.1086/283319) - DOI
-
- Maynard SJ. 1978. The evolution of sex. Cambridge, UK: Cambridge University Press.
-
- Vandel A. 1928. La parthénogenèse géographique: contribution a létude biologique et cytologique de la parthénogenèse naturelle [Geographical parthenogenesis: contribution to the biological and cytological study of natural parthenogenesis]. Bull. Biol. Fr. Belg. 62, 164–281.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
