Granulocyte Colony Stimulating Factor Enhances Reward Learning through Potentiation of Mesolimbic Dopamine System Function
- PMID: 30150359
- PMCID: PMC6181308
- DOI: 10.1523/JNEUROSCI.1116-18.2018
Granulocyte Colony Stimulating Factor Enhances Reward Learning through Potentiation of Mesolimbic Dopamine System Function
Abstract
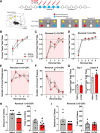
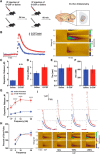
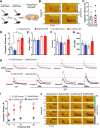
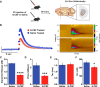
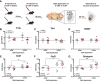
Deficits in motivation and cognition are hallmark symptoms of multiple psychiatric diseases. These symptoms are disruptive to quality of life and often do not improve with available medications. In recent years there has been increased interest in the role of the immune system in neuropsychiatric illness, but to date no immune-related treatment strategies have come to fruition. The cytokine granulocyte-colony stimulating factor (G-CSF) is known to have trophic and neuroprotective properties in the brain, and we recently identified it as a modulator of neuronal and behavioral plasticity. By combining operant tasks that assess discrete aspects of motivated behavior and decision-making in male mice and rats with subsecond dopamine monitoring via fast-scan cyclic voltammetry, we defined the role of G-CSF in these processes as well as the neural mechanism by which it modulates dopamine function to exert these effects. G-CSF enhanced motivation for sucrose as well as cognitive flexibility as measured by reversal learning. These behavioral outcomes were driven by mesolimbic dopamine system plasticity, as systemically administered G-CSF increased evoked dopamine release in the nucleus accumbens independent of clearance mechanisms. Importantly, sustained increases in G-CSF were required for these effects as acute exposure did not enhance behavioral outcomes and decreased dopamine release. These effects seem to be a result of the ability of G-CSF to alter local inflammatory signaling cascades, particularly tumor necrosis factor α. Together, these data show G-CSF as a potent modulator of the mesolimbic dopamine circuit and its ability to appropriately attend to salient stimuli.SIGNIFICANCE STATEMENT Emerging evidence has highlighted the importance of the immune system in psychiatric diseases states. However, the effects of peripheral cytokines on motivation and cognitive function are largely unknown. Here, we report that granulocyte-colony stimulating factor (G-CSF), a pleiotropic cytokine with known trophic and neuroprotective properties in the brain, acts directly on dopaminergic circuits to enhance their function. These changes in dopaminergic dynamics enhance reward learning and motivation for natural stimuli. Together, these results suggest that targeting immune factors may provide a new avenue for therapeutic intervention in the multiple psychiatric disorders that are characterized by motivational and cognitive deficits.
Keywords: cytokine; dopamine; immune system; learning and memory; motivation; voltammetry.
Copyright © 2018 the authors 0270-6474/18/388846-15$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
