Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption
- PMID: 30150398
- PMCID: PMC6140479
- DOI: 10.1073/pnas.1807105115
Mechanisms of enhanced drug delivery in brain metastases with focused ultrasound-induced blood-tumor barrier disruption
Abstract
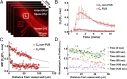
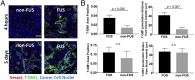
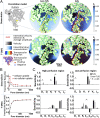
Blood-brain/blood-tumor barriers (BBB and BTB) and interstitial transport may constitute major obstacles to the transport of therapeutics in brain tumors. In this study, we examined the impact of focused ultrasound (FUS) in combination with microbubbles on the transport of two relevant chemotherapy-based anticancer agents in breast cancer brain metastases at cellular resolution: doxorubicin, a nontargeted chemotherapeutic, and ado-trastuzumab emtansine (T-DM1), an antibody-drug conjugate. Using an orthotopic xenograft model of HER2-positive breast cancer brain metastasis and quantitative microscopy, we demonstrate significant increases in the extravasation of both agents (sevenfold and twofold for doxorubicin and T-DM1, respectively), and we provide evidence of increased drug penetration (>100 vs. <20 µm and 42 ± 7 vs. 12 ± 4 µm for doxorubicin and T-DM1, respectively) after the application of FUS compared with control (non-FUS). Integration of experimental data with physiologically based pharmacokinetic (PBPK) modeling of drug transport reveals that FUS in combination with microbubbles alleviates vascular barriers and enhances interstitial convective transport via an increase in hydraulic conductivity. Experimental data demonstrate that FUS in combination with microbubbles enhances significantly the endothelial cell uptake of the small chemotherapeutic agent. Quantification with PBPK modeling reveals an increase in transmembrane transport by more than two orders of magnitude. PBPK modeling indicates a selective increase in transvascular transport of doxorubicin through small vessel wall pores with a narrow range of sizes (diameter, 10-50 nm). Our work provides a quantitative framework for the optimization of FUS-drug combinations to maximize intratumoral drug delivery and facilitate the development of strategies to treat brain metastases.
Keywords: blood–brain/blood–tumor barrier; brain tumor; drug transport; focused ultrasound; pharmacokinetics.
Conflict of interest statement
Conflict of interest statement: R.K.J. received an honorarium from Amgen and consultant fees from Merck, Ophthotech, Pfizer, SPARC, SynDevRx, and XTuit; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; served on the Board of Directors of XTuit; and serves on the Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, Tekla Healthcare Opportunities Fund, and Tekla World Healthcare Fund. Neither any reagent nor any funding from these organizations was used in this study.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

