Cyclin E1 and cyclin-dependent kinase 2 are critical for initiation, but not for progression of hepatocellular carcinoma
- PMID: 30150405
- PMCID: PMC6140539
- DOI: 10.1073/pnas.1807155115
Cyclin E1 and cyclin-dependent kinase 2 are critical for initiation, but not for progression of hepatocellular carcinoma
Abstract
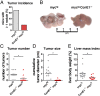
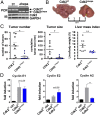
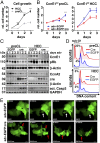
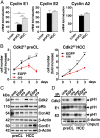
E-type cyclins E1 (CcnE1) and E2 (CcnE2) are regulatory subunits of cyclin-dependent kinase 2 (Cdk2) and thought to control the transition of quiescent cells into the cell cycle. Initial findings indicated that CcnE1 and CcnE2 have largely overlapping functions for cancer development in several tumor entities including hepatocellular carcinoma (HCC). In the present study, we dissected the differential contributions of CcnE1, CcnE2, and Cdk2 for initiation and progression of HCC in mice and patients. To this end, we tested the HCC susceptibility in mice with constitutive deficiency for CcnE1 or CcnE2 as well as in mice lacking Cdk2 in hepatocytes. Genetic inactivation of CcnE1 largely prevented development of liver cancer in mice in two established HCC models, while ablation of CcnE2 had no effect on hepatocarcinogenesis. Importantly, CcnE1-driven HCC initiation was dependent on Cdk2. However, isolated primary hepatoma cells typically acquired independence on CcnE1 and Cdk2 with increasing progression in vitro, which was associated with a gene signature involving secondary induction of CcnE2 and up-regulation of cell cycle and DNA repair pathways. Importantly, a similar expression profile was also found in HCC patients with elevated CcnE2 expression and poor survival. In general, overall survival in HCC patients was synergistically affected by expression of CcnE1 and CcnE2, but not through Cdk2. Our study suggests that HCC initiation specifically depends on CcnE1 and Cdk2, while HCC progression requires expression of any E-cyclin, but no Cdk2.
Keywords: DNA repair; HCC; cell cycle; diethylnitrosamine; liver.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

