Evaluation of MEDI8852, an Anti-Influenza A Monoclonal Antibody, in Treating Acute Uncomplicated Influenza
- PMID: 30150460
- PMCID: PMC6201130
- DOI: 10.1128/AAC.00694-18
Evaluation of MEDI8852, an Anti-Influenza A Monoclonal Antibody, in Treating Acute Uncomplicated Influenza
Abstract
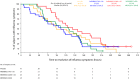
We evaluated MEDI8852, a human IgG1 monoclonal antibody that binds a highly conserved influenza A hemagglutinin stalk epitope, in outpatients with uncomplicated influenza A infection. A total of 126 subjects aged 18 to 65 years were enrolled during the 2015 to 2016 Northern and 2016 Southern Hemisphere seasons. Subjects with symptom onset ≤5 days before dosing were randomized to four cohorts: 750 mg (cohort 1) or 3,000 mg (cohort 2) MEDI8852 (single intravenous infusion) plus 75 mg oseltamivir, placebo plus 75 mg oseltamivir (cohort 3), and 3,000 mg MEDI8852 alone (cohort 4). Subjects were monitored through day 10 for solicited influenza symptoms, day 28 for adverse events (AEs), and day 101 for serious AEs and AEs of special interest. Nasopharyngeal samples were collected through day 7 for confirmation of influenza A infection, viral shedding, and oseltamivir and MEDI8852 susceptibility. Slightly more AEs were reported in subjects receiving MEDI8852 (cohorts 1, 2, and 4 combined: 39/93, 41.9%) than oseltamivir only (cohort 3: 10/32, 31.3%). Most AEs were mild or moderate. The most common AE was bronchitis (11/93, 11.8%; 1/32, 3.1%). The median (range) decrease in viral shedding (log10 virus genome copies/ml) was similar between the two groups (-3.58 [-6.2. 0.5]; -3.43 [-5.9, 0.9]). Genotypic analyses found a limited number of hemagglutinin and neuraminidase amino acid changes between viruses isolated before and after therapy; however, none appeared within a known oseltamivir-resistant site or MEDI8852-binding region. The safety profile of MEDI8852 supports its continued development for treatment of patients hospitalized with influenza A infection. (This study has been registered at ClinicalTrials.gov under identifier NCT02603952.).
Keywords: influenza; monoclonal antibody; safety; treatment.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Centers for Disease Control and Prevention. 2017. Summary of the 2015-2016 influenza season. US Department of Health and Human Services, Washington, DC: https://www.cdc.gov/flu/about/season/flu-season-2015-2016.htm.
-
- Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM, Centers for Disease Control and Prevention . 2011. Antiviral agents for the treatment and chemoprophylaxis of influenza—recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 60:1–24. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

