Randomized, Double-Blind, Placebo-Controlled Studies of the Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Eravacycline
- PMID: 30150464
- PMCID: PMC6201080
- DOI: 10.1128/AAC.01174-18
Randomized, Double-Blind, Placebo-Controlled Studies of the Safety and Pharmacokinetics of Single and Multiple Ascending Doses of Eravacycline
Abstract
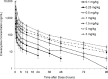
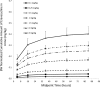
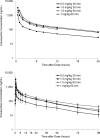
Eravacycline is a novel, fully synthetic fluorocycline antibiotic with in vitro activity against aerobic and anaerobic Gram-positive and Gram-negative pathogens, including multidrug-resistant (MDR) bacteria. The pharmacokinetics (PK), urinary excretion, and safety/tolerability of intravenous (i.v.) eravacycline were evaluated in single- and multiple-ascending-dose studies. Healthy subjects received single i.v. doses of 0.1 to 3 mg/kg of body weight or 10 days of treatment with 0.5 or 1.5 mg/kg every 24 h (q24h) over 30 min, 1.5 mg/kg q24h over 60 min, or 1 mg/kg q12h over 60 min. After single doses, total exposure (the area under the plasma concentration-time curve [AUC]) and the maximum plasma concentrations (Cmax) of eravacycline increased in an approximately dose-proportional manner. After multiple doses, steady state was achieved within 5 to 7 days. Accumulation ranged from approximately 7% to 38% with the q24h dosing regimens and was 45% with 1 mg/kg q12h. Eravacycline was generally well tolerated, with dose-related nausea, infusion site effects, and superficial phlebitis that were mild or moderate occurring. These results provide support for the 1-mg/kg q12h regimen used in clinical studies of eravacycline.
Keywords: eravacycline; pharmacokinetics.
Copyright © 2018 Newman et al.
Figures

References
-
- Abdallah M, Olafisoye O, Cortes C, Urban C, Landman D, Quale J. 2015. Activity of eravacycline against Enterobacteriaceae and Acinetobacter baumannii, including multidrug-resistant isolates, from New York City. Antimicrob Agents Chemother 59:1802–1805. doi: 10.1128/AAC.04809-14. - DOI - PMC - PubMed
-
- Johnson JR, Porter SB, Johnston BD, Thuras P. 2015. Activity of eravacycline against Escherichia coli clinical isolates collected from U.S. veterans in 2011 in relation to coresistance phenotype and sequence type 131 genotype. Antimicrob Agents Chemother 60:1888–1891. doi: 10.1128/AAC.02403-15. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

