Macrophage Immunomodulation: The Gatekeeper for Mesenchymal Stem Cell Derived-Exosomes in Pulmonary Arterial Hypertension?
- PMID: 30150544
- PMCID: PMC6164282
- DOI: 10.3390/ijms19092534
Macrophage Immunomodulation: The Gatekeeper for Mesenchymal Stem Cell Derived-Exosomes in Pulmonary Arterial Hypertension?
Abstract
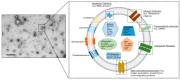
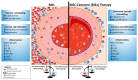
Pulmonary arterial hypertension (PAH) is a progressive disease characterized by remodeling of the pulmonary arteries, increased pulmonary infiltrates, loss of vascular cross-sectional area, and elevated pulmonary vascular resistance. Despite recent advances in the management of PAH, there is a pressing need for the development of new tools to effectively treat and reduce the risk of further complications. Dysregulated immunity underlies the development of PAH, and macrophages orchestrate both the initiation and resolution of pulmonary inflammation, thus, manipulation of lung macrophage function represents an attractive target for emerging immunomodulatory therapies, including cell-based approaches. Indeed, mesenchymal stem cell (MSC)-based therapies have shown promise, effectively modulating the macrophage fulcrum to favor an anti-inflammatory, pro-resolving phenotype, which is associated with both histological and functional benefits in preclinical models of pulmonary hypertension (PH). The complex interplay between immune system homeostasis and MSCs remains incompletely understood. Here, we highlight the importance of macrophage function in models of PH and summarize the development of MSC-based therapies, focusing on the significance of MSC exosomes (MEx) and the immunomodulatory and homeostatic mechanisms by which such therapies may afford their beneficial effects.
Keywords: MSC exosomes (MEx); bronchopulmonary dysplasia (BPD); exosomes; extracellular vesicles (EVs); inflammation; macrophages; mesenchymal stem cells (MSCs); pulmonary arterial hypertension (PAH); pulmonary hypertension (PH).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation.Am J Respir Crit Care Med. 2018 Jan 1;197(1):104-116. doi: 10.1164/rccm.201705-0925OC. Am J Respir Crit Care Med. 2018. PMID: 28853608 Free PMC article.
-
Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice.Cardiovasc Res. 2016 Jun 1;110(3):319-30. doi: 10.1093/cvr/cvw054. Epub 2016 Mar 14. Cardiovasc Res. 2016. PMID: 26980205 Free PMC article.
-
The Unique Immunomodulatory Properties of MSC-Derived Exosomes in Organ Transplantation.Front Immunol. 2021 Apr 6;12:659621. doi: 10.3389/fimmu.2021.659621. eCollection 2021. Front Immunol. 2021. PMID: 33889158 Free PMC article. Review.
-
Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension.Circulation. 2012 Nov 27;126(22):2601-11. doi: 10.1161/CIRCULATIONAHA.112.114173. Epub 2012 Oct 31. Circulation. 2012. PMID: 23114789 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles Therapy for Pulmonary Hypertension: A Comprehensive Review of Preclinical Studies.J Interv Cardiol. 2022 Nov 4;2022:5451947. doi: 10.1155/2022/5451947. eCollection 2022. J Interv Cardiol. 2022. PMID: 36419957 Free PMC article. Review.
Cited by
-
Activation of Nicotinic Acetylcholine α7 Receptor Attenuates Progression of Monocrotaline-Induced Pulmonary Hypertension in Rats by Downregulating the NLRP3 Inflammasome.Front Pharmacol. 2019 Feb 26;10:128. doi: 10.3389/fphar.2019.00128. eCollection 2019. Front Pharmacol. 2019. PMID: 30863307 Free PMC article.
-
Advances in mesenchymal stem cells therapy for tendinopathies.Chin J Traumatol. 2024 Jan;27(1):11-17. doi: 10.1016/j.cjtee.2023.11.002. Epub 2023 Nov 11. Chin J Traumatol. 2024. PMID: 38052701 Free PMC article. Review.
-
Mesenchymal stem cell-derived extracellular vesicles prevent the formation of pulmonary arterial hypertension through a microRNA-200b-dependent mechanism.Respir Res. 2023 Sep 27;24(1):233. doi: 10.1186/s12931-023-02474-7. Respir Res. 2023. PMID: 37759281 Free PMC article.
-
HNRNPA2B1: a novel target in pulmonary arterial hypertension.Front Cardiovasc Med. 2025 Jul 9;12:1497938. doi: 10.3389/fcvm.2025.1497938. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40703632 Free PMC article. Review.
-
Mechanotransduction Regulates the Interplays Between Alveolar Epithelial and Vascular Endothelial Cells in Lung.Front Physiol. 2022 Feb 18;13:818394. doi: 10.3389/fphys.2022.818394. eCollection 2022. Front Physiol. 2022. PMID: 35250619 Free PMC article. Review.
References
-
- Tuder R.M., Archer S.L., Dorfmüller P., Erzurum S.C., Guignabert C., Michelakis E., Rabinovitch M., Schermuly R., Stenmark K.R., Morrell N.W. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J. Am. Coll. Cardiol. 2013;62:D4–D12. doi: 10.1016/j.jacc.2013.10.025. - DOI - PMC - PubMed
-
- Morrell N.W., Adnot S., Archer S.L., Dupuis J., Jones P.L., MacLean M.R., McMurtry I.F., Stenmark K.R., Thistlethwaite P.A., Weissmann N., et al. Cellular and molecular basis of pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2009;54:S20–S31. doi: 10.1016/j.jacc.2009.04.018. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

