TiO₂ Nanotubes/Ag/MoS₂ Meshy Photoelectrode with Excellent Photoelectrocatalytic Degradation Activity for Tetracycline Hydrochloride
- PMID: 30150575
- PMCID: PMC6163688
- DOI: 10.3390/nano8090666
TiO₂ Nanotubes/Ag/MoS₂ Meshy Photoelectrode with Excellent Photoelectrocatalytic Degradation Activity for Tetracycline Hydrochloride
Abstract
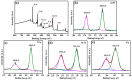
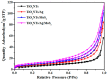
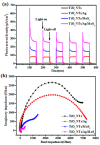
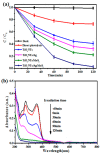
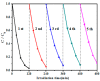
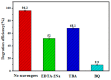
A novel type of TiO₂ nanotubes (NTs)/Ag/MoS₂ meshy photoelectrode was fabricated with highly oriented TiO₂ nanotube arrays grown from a Ti mesh supporting Ag nanoparticles and three-dimensional MoS₂ nanosheets. In this structure, Ag nanoparticles act as bridges to connect MoS₂ and TiO₂ and pathways for electron transfer, ensuring the abundant production of active electrons, which are the source of •O₂-. The TiO₂ NTs/Ag/MoS₂ mesh can be used as both photocatalyst and electrode, exhibiting enhanced photoelectrocatalytic efficiency in degrading tetracycline hydrochloride under visible light irradiation (λ ≥ 420 nm). Compared to unmodified TiO₂ NTs, the improved photoelectrocatalytic activity of the TiO₂ NTs/Ag/MoS₂ arise from the formation of Z-scheme heterojunctions, which facilitate the efficient separation of photogenerated electron-hole pairs through the Schottky barriers at the interfaces of TiO₂ NTs⁻Ag and Ag⁻MoS₂.
Keywords: TiO2 nanotubes/Ag/MoS2; photoelectrocatalytic degradation; photoelectrode; tetracycline hydrochloride.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






Similar articles
-
Photoelectrocatalytic degradation of organic pollutants via a CdS quantum dots enhanced TiO2 nanotube array electrode under visible light irradiation.Nanoscale. 2013 Mar 7;5(5):2118-25. doi: 10.1039/c3nr34253k. Epub 2013 Feb 5. Nanoscale. 2013. PMID: 23381869
-
Influence of Ag-Au microstructure on the photoelectrocatalytic performance of TiO2 nanotube array photocatalysts.J Colloid Interface Sci. 2016 Feb 1;463:308-16. doi: 10.1016/j.jcis.2015.10.063. Epub 2015 Oct 28. J Colloid Interface Sci. 2016. PMID: 26555961
-
Electrochemically assisted photocatalytic degradation of 4-chlorophenol by ZnFe2O4-modified TiO2 nanotube array electrode under visible light irradiation.Environ Sci Technol. 2010 Jul 1;44(13):5098-103. doi: 10.1021/es100004u. Environ Sci Technol. 2010. PMID: 20527761
-
Hierarchical top-porous/bottom-tubular TiO2 nanostructures decorated with Pd nanoparticles for efficient Photoelectrocatalytic decomposition of synergistic pollutants.ACS Appl Mater Interfaces. 2012 Feb;4(2):990-6. doi: 10.1021/am201630s. Epub 2012 Jan 24. ACS Appl Mater Interfaces. 2012. PMID: 22233777
-
Solvothermal fabrication and construction of highly photoelectrocatalytic TiO2 NTs/Bi2MoO6 heterojunction based on titanium mesh.J Colloid Interface Sci. 2019 Nov 15;556:92-101. doi: 10.1016/j.jcis.2019.08.038. Epub 2019 Aug 9. J Colloid Interface Sci. 2019. PMID: 31430709
Cited by
-
Black 3D-TiO2 Nanotube Arrays on Ti Meshes for Boosted Photoelectrochemical Water Splitting.Nanomaterials (Basel). 2022 Apr 24;12(9):1447. doi: 10.3390/nano12091447. Nanomaterials (Basel). 2022. PMID: 35564156 Free PMC article.
-
A three-dimensional nano-network WO3/F-TiO2-{001} heterojunction constructed with OH-TiOF2 as the precursor and its efficient degradation of methylene blue.RSC Adv. 2021 Jul 29;11(42):26063-26072. doi: 10.1039/d1ra04809k. eCollection 2021 Jul 27. RSC Adv. 2021. PMID: 35479479 Free PMC article.
-
Titanium dioxide modified with silver by two methods for bactericidal applications.Heliyon. 2019 May 14;5(5):e01608. doi: 10.1016/j.heliyon.2019.e01608. eCollection 2019 May. Heliyon. 2019. PMID: 31193210 Free PMC article.
-
The Roles of Nanomaterials in Conventional and Emerging Technologies for Heavy Metal Removal: A State-of-the-Art Review.Nanomaterials (Basel). 2019 Apr 17;9(4):625. doi: 10.3390/nano9040625. Nanomaterials (Basel). 2019. PMID: 30999639 Free PMC article. Review.
-
Photoelectrochemical water oxidation over TiO2 nanotubes modified with MoS2 and g-C3N4.Beilstein J Nanotechnol. 2022 Dec 16;13:1541-1550. doi: 10.3762/bjnano.13.127. eCollection 2022. Beilstein J Nanotechnol. 2022. PMID: 36605609 Free PMC article.
References
-
- Sui Q., Cao X.Q., Lu S.G., Zhao W.T., Qiu Z.F., Yu G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015;1:14–24. doi: 10.1016/j.emcon.2015.07.001. - DOI
-
- He D., Sun Y.B., Xin L., Feng J.W. Aqueous tetracycline degradation by non-thermal plasma combined with nano-TiO2. Chem. Eng. J. 2014;258:18–25. doi: 10.1016/j.cej.2014.07.089. - DOI
-
- Chen F., Yang Q., Li X.M., Zeng G.M., Wang D.B., Niu C.G., Zhao J.W., An H.X., Xie T., Deng Y.C. Hierarchical assembly of graphene-bridged Ag3PO4/Ag/ BiVO4(040) Z-scheme photocatalyst: An efficient, sustainable and heterogeneous catalyst with enhanced visible-light photoactivity towards tetracycline degradation under visible light irradiation. Appl. Catal. B Environ. 2017;200:330–342. doi: 10.1016/j.apcatb.2016.07.021. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

