LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation
- PMID: 30150626
- PMCID: PMC6110743
- DOI: 10.1038/s41467-018-05958-z
LRRK2 kinase regulates α-synuclein propagation via RAB35 phosphorylation
Abstract
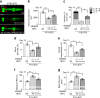
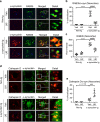
Propagation of α-synuclein aggregates has been suggested as a contributing factor in Parkinson's disease (PD) progression. However, the molecular mechanisms underlying α-synuclein aggregation are not fully understood. Here, we demonstrate in cell culture, nematode, and rodent models of PD that leucine-rich repeat kinase 2 (LRRK2), a PD-linked kinase, modulates α-synuclein propagation in a kinase activity-dependent manner. The PD-linked G2019S mutation in LRRK2, which increases kinase activity, enhances propagation efficiency. Furthermore, we show that the role of LRRK2 in α-synuclein propagation is mediated by RAB35 phosphorylation. Constitutive activation of RAB35 overrides the reduced α-synuclein propagation phenotype in lrk-1 mutant C. elegans. Finally, in a mouse model of synucleinopathy, administration of an LRRK2 kinase inhibitor reduced α-synuclein aggregation via enhanced interaction of α-synuclein with the lysosomal degradation pathway. These results suggest that LRRK2-mediated RAB35 phosphorylation is a potential therapeutic target for modifying disease progression.
Conflict of interest statement
S.J.L. received research grants from and is a stock-holder of Abl Bio. The remaining authors declare no competing interests.
Figures

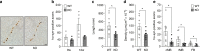
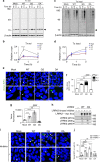
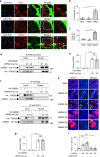

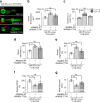

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

