The effect of maternal care on gene expression and DNA methylation in a subsocial bee
- PMID: 30150650
- PMCID: PMC6110825
- DOI: 10.1038/s41467-018-05903-0
The effect of maternal care on gene expression and DNA methylation in a subsocial bee
Abstract
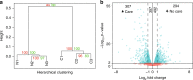
Developmental plasticity describes the influence of environmental factors on phenotypic variation. An important mediator of developmental plasticity in many animals is parental care. Here, we examine the consequences of maternal care on offspring after the initial mass provisioning of brood in the small carpenter bee, Ceratina calcarata. Removal of the mother during larval development leads to increased aggression and avoidance in adulthood. This corresponds with changes in expression of over one thousand genes, alternative splicing of hundreds of genes, and significant changes to DNA methylation. We identify genes related to metabolic and neuronal functions that may influence developmental plasticity and aggression. We observe no genome-wide association between differential DNA methylation and differential gene expression or splicing, though indirect relationships may exist between these factors. Our results provide insight into the gene regulatory context of DNA methylation in insects and the molecular avenues through which variation in maternal care influences developmental plasticity.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
The Genome and Methylome of a Subsocial Small Carpenter Bee, Ceratina calcarata.Genome Biol Evol. 2016 May 13;8(5):1401-10. doi: 10.1093/gbe/evw079. Genome Biol Evol. 2016. PMID: 27048475 Free PMC article.
-
At the brink of eusociality: transcriptomic correlates of worker behaviour in a small carpenter bee.BMC Evol Biol. 2014 Dec 17;14:260. doi: 10.1186/s12862-014-0260-6. BMC Evol Biol. 2014. PMID: 25514967 Free PMC article.
-
Defense against territorial intrusion is associated with DNA methylation changes in the honey bee brain.BMC Genomics. 2018 Mar 26;19(1):216. doi: 10.1186/s12864-018-4594-0. BMC Genomics. 2018. PMID: 29580210 Free PMC article.
-
DNA Methylation and Gene Regulation in Honeybees: From Genome-Wide Analyses to Obligatory Epialleles.Adv Exp Med Biol. 2016;945:193-211. doi: 10.1007/978-3-319-43624-1_9. Adv Exp Med Biol. 2016. PMID: 27826840 Review.
-
Understanding the relationship between brain gene expression and social behavior: lessons from the honey bee.Annu Rev Genet. 2012;46:591-615. doi: 10.1146/annurev-genet-110711-155517. Epub 2012 Sep 17. Annu Rev Genet. 2012. PMID: 22994354 Review.
Cited by
-
Intergenerational transfer of DNA methylation marks in the honey bee.Proc Natl Acad Sci U S A. 2020 Dec 22;117(51):32519-32527. doi: 10.1073/pnas.2017094117. Epub 2020 Nov 30. Proc Natl Acad Sci U S A. 2020. PMID: 33257552 Free PMC article.
-
Exploring the genetics of social behaviour in C. calcarata.Sci Rep. 2025 Feb 15;15(1):5580. doi: 10.1038/s41598-025-89870-9. Sci Rep. 2025. PMID: 39955334 Free PMC article.
-
Unravelling the role of epigenetic regulators during embryonic development of Rhipicephalus microplus.bioRxiv [Preprint]. 2025 Jul 11:2025.07.11.662657. doi: 10.1101/2025.07.11.662657. bioRxiv. 2025. PMID: 40672298 Free PMC article. Preprint.
-
Bumblebee Workers Show Differences in Allele-Specific DNA Methylation and Allele-Specific Expression.Genome Biol Evol. 2020 Aug 1;12(8):1471-1481. doi: 10.1093/gbe/evaa132. Genome Biol Evol. 2020. PMID: 32597949 Free PMC article.
-
Insects Provide Unique Systems to Investigate How Early-Life Experience Alters the Brain and Behavior.Front Behav Neurosci. 2021 Apr 21;15:660464. doi: 10.3389/fnbeh.2021.660464. eCollection 2021. Front Behav Neurosci. 2021. PMID: 33967715 Free PMC article. Review.
References
-
- West-Eberhard, M. J. Developmental Plasticity and Evolution (Oxford University Press, 2003).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

