Linking host morphology and symbiont performance in octocorals
- PMID: 30150781
- PMCID: PMC6110782
- DOI: 10.1038/s41598-018-31262-3
Linking host morphology and symbiont performance in octocorals
Abstract
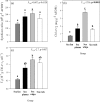
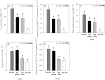
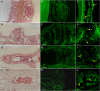
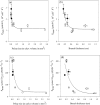
Octocorals represent an important group in reef communities throughout the tropical seas and, like scleractinian corals, they can be found in symbiosis with the dinoflagellate Symbiodinium. However, while there is extensive research on this symbiosis and its benefits in scleractinians, research on octocorals has focused so far mainly on the host without addressing their symbiosis. Here, we characterized and compared the photophysiological features of nine Caribbean octocoral species with different colony morphologies (sea fan, plumes, whips and rods) and related key morphological features with their respective symbiont photobiology. Colony features (branch shape and thickness), as well as micromorphological features (polyp size, density), were found to be significantly correlated with symbiont performance. Sea fans and plumes, with thinner branches and smaller polyps, favor higher metabolic rates, compared to sea rods with thicker branches and larger polyps. Daily integrated photosynthesis to respiration ratios > 1 indicated that the autotrophic contribution to organisms' energy demands was important in all species, but especially in sea whips. This information represents an important step towards a better understanding of octocoral physiology and its relationship to host morphology, and might also explain to some extent species distribution and susceptibility to environmental stress.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Wild C, et al. Climate change impedes scleractinian corals as primary reef ecosystem engineers. Mar. Freshw. Res. 2011;62:205–215. doi: 10.1071/MF10254. - DOI
-
- Williams, G. C. & Cairns, S. D. Biodiversity myth busters, Octocoral Research Center. http://researcharchive.calacademy.org/research/izg/Biodiversity%20Myth%2... (2013).
-
- Sánchez, J. A. Diversity and evolution of octocoral animal forests at both sides of tropical America in Marine animal forests (eds Rossi, S., Bramanti, L., Gori, A. & Orejas, C.) 1–33 (2016).
-
- Schubert, N., Brown, D. & Rossi, S. Symbiotic versus non-symbiotic octocorals: Physiological and ecological implications in Marine animal forests (eds Rossi, S., Bramanti, L., Gori, A. & Orejas, C.) 887–918 (2017).
-
- Stambler, N. Zooxanthellae: the yellow symbionts inside animals in Coral Reefs: an ecosystem in transition (eds Dubinsky, Z. & Stambler, N.) 87–106 (2011).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

