Interleukin-6 and Type-I Collagen Production by Systemic Sclerosis Fibroblasts Are Differentially Regulated by Interleukin-17A in the Presence of Transforming Growth Factor-Beta 1
- PMID: 30150989
- PMCID: PMC6099180
- DOI: 10.3389/fimmu.2018.01865
Interleukin-6 and Type-I Collagen Production by Systemic Sclerosis Fibroblasts Are Differentially Regulated by Interleukin-17A in the Presence of Transforming Growth Factor-Beta 1
Abstract
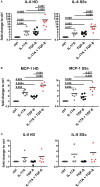
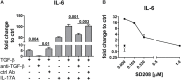
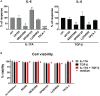
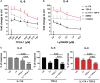
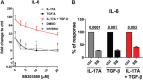
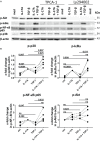
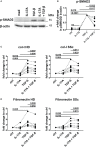
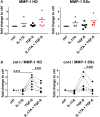
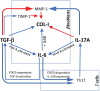
Functional cytokine networks have been poorly characterized in systemic sclerosis (SSc). While interleukin-17A (IL-17A) is increased in SSc skin and other organs, its role is still debated, particularly considering fibrogenesis. We uncover here a dual function of IL-17A in the presence of transforming growth factor-β 1 (TGF-β), the master pro-fibrotic cytokine. In the one hand, we report an unexpected synergic activity resulting in enhanced production of IL-6 by dermal fibroblasts; in the other hand, a substantial inhibition of type I collagen (col-I) production. IL-17A or TGF-β enhanced the production of IL-6 by 8- to 16-folds when compared to control in healthy donors (HD) and SSc cultures. However, the joint presence of IL-17A and TGF-β resulted in robustly exuberant responses with levels of IL-6 up to 100-folds higher than those observed in untreated cells. Inhibition of NFκB signaling pathway preferentially inhibited the production of IL-6 driven by IL-17A in HD fibroblasts, while inhibition of PI3K preferentially inhibited the production of IL-6 driven by TGF-β. Interestingly, when p38 MAPK was inhibited, substantial reduction of IL-6 production was observed for both IL-17A and TGF-β. Consistently with the inhibition experiments, the combined stimulation of fibroblasts by IL-17A and TGF-β resulted in 1.8-fold increase in p38 MAPK phosphorylation (P = 0.025), when compared to levels of phosphorylated p38 MAPK induced by IL-17A alone. Furthermore, the enhanced phosphorylation of p38 MAPK in the joint presence of IL-17A and TGF-β was unique among the signaling molecules we examined. As expected, TGF-β induced SMAD2 phosphorylation and col-I production. However, in fibroblasts cultured in the joint presence of TGF-β and IL-17A, SMAD2 phosphorylation was decreased by 0.6-folds (P = 0.022) when compared to that induced by TGF-β alone. Remarkably, in this condition, the production of col-I and fibronectin was significantly decreased in both HD and SSc. Thus, IL-17A and TGF-β reciprocally influence each other effector functions in fibroblasts. Intracellular molecular switches may favor synergic or antagonistic activities, which are revealed by specific readouts. The implications of these data in the context of SSc are far reaching, particularly in terms of therapeutic approaches since IL-6, IL-17A, and TGF-β are all putative targets of treatment.
Keywords: fibrosis; interleukin-17A; interleukin-6; monocyte chemotactic protein-1; systemic sclerosis; transforming growth factor-beta; type-I collagen.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

