Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition
- PMID: 30151257
- PMCID: PMC6087857
- DOI: 10.21037/jgo.2018.05.06
Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition
Abstract
Background: The clinical application of PD1/PD-L1 targeting checkpoint inhibitors in colorectal cancer (CRC) has largely focused on a subset of microsatellite instable (MSI-high) patients. However, the proposed genotype that sensitizes these patients to immunotherapy is not captured by MSI status alone. Estimation of tumor mutational burden (TMB) from comprehensive genomic profiling is validated against whole exome sequencing and linked to checkpoint response in metastatic melanoma, urothelial bladder cancer and non-small cell lung carcinoma. We sought to explore the subset of microsatellite stable (MSS) CRC patients with high TMB, and identify the specific genomic signatures associated with this phenotype. Furthermore, we explore the ability to quantify TMB as a potential predictive biomarker of PD1/PD-L1 therapy in CRC.
Methods: Formalin-fixed, paraffin embedded tissue sections from 6,004 cases of CRC were sequenced with a CLIA-approved CGP assay. MSI and TMB statuses were computationally determined using validated methods. The cutoff for TMB-high was defined according to the lower bound value that satisfied the 90% probability interval based on the TMB distribution across all MSI-High patients.
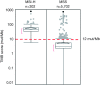
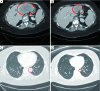
Results: MSS tumors were observed in 5,702 of 6,004 (95.0%) cases and MSI-H tumors were observed in 302 (5.0%) cases. All but one (99.7%) MSI-H cases were TMB-high (range, 6.3-746.9 mut/Mb) and 5,538 of 5,702 (97.0%) MSS cases were TMB-low (range, 0.0-10.8 mut/Mb). Consequently, 164 of 5,702 (2.9%) MSS cases were confirmed as TMB-high (range, 11.7-707.2 mut/Mb), representing an increase in the target population that may respond to checkpoint inhibitor therapy by 54% (466 vs. 302, respectively). Response to PD-1 inhibitor is demonstrated in MSS/TMB-high cases.
Conclusions: Concurrent TMB assessment accurately classifies MSI tumors as TMB-high and simultaneously identifies nearly 3% or CRC as MSS/TMB-high. This subgroup may expand the population of CRC who may benefit from immune checkpoint inhibitor based therapeutic approaches.
Keywords: PD-1; colorectal cancer; immunotherapy; microsatellite instability; tumor mutational burden.
Conflict of interest statement
Conflicts of Interest: DA Fabrizio, G Frampton, J Sun, K Gowen, M Kennedy, J Greenbowe, AB Schrock, JS Ross, PJ Stephens, SM Ali, and VA Miller are employees and hold equity in Foundation Medicine, Inc. DA Fabrizio holds equity in Juno Therapeutics and Seattle Genetics. SJ Klempner has received honoraria from Foundation Medicine, Inc. The other authors have no conflicts of interest to declare.
Figures
References
-
- Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. 10.1016/S1470-2045(14)70330-4 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
