Mechanically induced development and maturation of human intestinal organoids in vivo
- PMID: 30151330
- PMCID: PMC6108544
- DOI: 10.1038/s41551-018-0243-9
Mechanically induced development and maturation of human intestinal organoids in vivo
Abstract
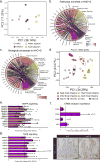
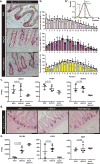
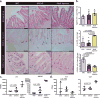
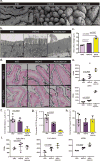
The natural ability of stem cells to self-organize into functional tissue has been harnessed for the production of functional human intestinal organoids. Although dynamic mechanical forces play a central role in intestinal development and morphogenesis, conventional methods for the generation of intestinal organoids have relied solely on biological factors. Here, we show that the incorporation of uniaxial strain, by using compressed nitinol springs, in human intestinal organoids transplanted into the mesentery of mice induces growth and maturation of the organoids. Assessment of morphometric parameters, transcriptome profiling, and functional assays of the strain-exposed tissue revealed higher similarities to native human intestine, with regards to tissue size and complexity, and muscle tone. Our findings suggest that the incorporation of physiologically relevant mechanical cues during the development of human intestinal tissue enhances its maturation and enterogenesis.
Keywords: Human Pluripotent Stem Cells; Small Intestine; Tissue Engineering; Transplantation; Uniaxial Strain.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

