Ouabain Does Not Induce Selective Spiral Ganglion Cell Degeneration in Guinea Pigs
- PMID: 30151372
- PMCID: PMC6091334
- DOI: 10.1155/2018/1568414
Ouabain Does Not Induce Selective Spiral Ganglion Cell Degeneration in Guinea Pigs
Abstract
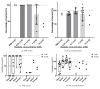
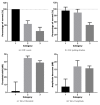
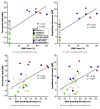
Round window membrane (RWM) application of ouabain is known to selectively destroy type I spiral ganglion cells (SGCs) in cochleas of several rodent species, while leaving hair cells intact. This protocol has been used in rats and Mongolian gerbils, but observations in the guinea pig are conflicting. This is why we reinvestigated the effect of ouabain on the guinea pig cochlea. Ouabain solutions of different concentrations were placed, in a piece of gelfoam, upon the RWM of the right cochleas. Auditory function was assessed using acoustically evoked auditory brainstem responses (aABR). Finally, cochleas were fixed and processed for histological examination. Due to variability within treatment groups, histological data was pooled and three categories based upon general histological observations were defined: cochleas without outer hair cell (OHC) and SGC loss (Category 1), cochleas with OHC loss only (Category 2), and cochleas with OHC and SGC loss (Category 3). Animals treated with 1 mM or 10 mM ouabain showed shifts in hearing thresholds, corresponding with varying histological changes in their cochleas. Most cochleas exhibited complete outer hair cell loss in the basal and middle turns, while some had no changes, together with either moderate or near-complete loss of SGCs. Neither loss of inner hair cells nor histological changes of the stria vascularis were observed in any of the animals. Cochleas in Category 1 had normal aABRs and morphology. On average, in Category 2 OHC loss was 46.0±5.7%, SGC loss was below threshold, ABR threshold shift was 44.9±2.7 dB, and ABR wave II amplitude was decreased by 17.1±3.8 dB. In Category 3 OHC loss was 68.3±6.9%, SGC loss was 49.4±4.3%, ABR threshold shift was 39.0±2.4 dB, and ABR amplitude was decreased by 15.8±1.6 dB. Our results show that ouabain does not solely destroy type I SGCs in the guinea pig cochlea.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

