Adverse side effects of dexamethasone in surgical patients
- PMID: 30152137
- PMCID: PMC6513495
- DOI: 10.1002/14651858.CD011940.pub2
Adverse side effects of dexamethasone in surgical patients
Update in
-
Adverse side effects of dexamethasone in surgical patients.Cochrane Database Syst Rev. 2018 Nov 23;11(11):CD011940. doi: 10.1002/14651858.CD011940.pub3. Cochrane Database Syst Rev. 2018. PMID: 30480776 Free PMC article.
Abstract
Background: In the perioperative period, dexamethasone is widely and effectively used for prophylaxis of postoperative nausea and vomiting (PONV), for pain management, and to facilitate early discharge after ambulatory surgery.Long-term treatment with steroids has many side effects, such as adrenal insufficiency, increased infection risk, hyperglycaemia, high blood pressure, osteoporosis, and development of diabetes mellitus. However, whether a single steroid load during surgery has negative effects during the postoperative period has not yet been studied.
Objectives: To assess the effects of a steroid load of dexamethasone on postoperative systemic or wound infection, delayed wound healing, and blood glucose change in adult surgical patients (with planned subgroup analysis of patients with and without diabetes).
Search methods: We searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials (CENTRAL), in the Cochrane Library, and the Web of Science for relevant articles on 29 January 2018. We searched without language or date restriction two clinical trial registries to identify ongoing studies, and we handsearched the reference lists of relevant publications to identify all eligible trials.
Selection criteria: We searched for randomized controlled trials comparing an incidental steroid load of dexamethasone versus a control intervention for adult patients undergoing surgery. We required that studies include a follow-up of 30 days for proper assessment of the number of postoperative infections, delayed wound healing, and the glycaemic response.
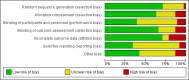
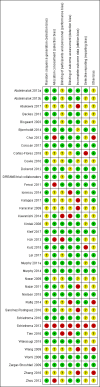
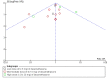
Data collection and analysis: Two review authors independently screened studies for eligibility, extracted data from relevant studies, and assessed all included studies for bias. We resolved differences by discussion and pooled included studies in a meta-analysis. We calculated Peto odds ratios (ORs) for dichotomous outcomes and mean differences (MDs) for continuous outcomes. Our primary outcomes were postoperative systemic or wound infection, delayed wound healing, and glycaemic response within 24 hours. We created a funnel plot for the primary outcome postoperative (wound or systemic) infection. We used GRADE to assess the quality of evidence for each outcome.
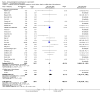
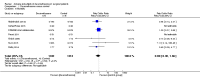
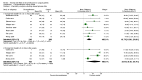
Main results: We included in the meta-analysis 38 studies that included adults undergoing a large variety of surgical procedures (i.e. abdominal surgery, cardiac surgery, neurosurgery, and orthopaedic surgery). Age range of participants was 18 to 80 years. There is probably little or no difference in the risk of postoperative (wound or systemic) infection with dexamethasone compared with no treatment, placebo, or active control (ramosetron, ondansetron, or tropisetron) (Peto OR 1.01, 95% confidence interval (CI) 0.80 to 1.27; 4931 participants, 27 studies; I² = 27%; moderate-quality evidence). The effects of dexamethasone on delayed wound healing are unclear because the wide confidence interval includes both meaningful benefit and harm (Peto OR 0.99, 95% CI 0.28 to 3.43; 1072 participants, eight studies; I² = 0%; low-quality evidence). Dexamethasone may produce a mild increase in glucose levels among participants without diabetes during the first 12 hours after surgery (MD 13 mg/dL, 95% CI 6 to 21; 10 studies; 595 participants; I² = 50%; low-quality evidence). We identified two studies reporting on glycaemic response after dexamethasone in participants with diabetes within 24 hours after surgery (MD 32 mg/dL, 95% CI 15 to 49; 74 participants; I² = 0%; very low-quality evidence).
Authors' conclusions: A single dose of dexamethasone probably does not increase the risk for postoperative infection. It is uncertain whether dexamethasone has an effect on delayed wound healing in the general surgical population owing to imprecision in trial results. Participants with increased risk for delayed wound healing (e.g. participants with diabetes, those taking immunosuppressive drugs) were not included in the randomized studies reporting on delayed wound healing included in this meta-analysis; therefore our findings should be extrapolated to the clinical setting with caution. Furthermore, one has to keep in mind that dexamethasone induces a mild increase in glucose. For patients with diabetes, very limited evidence suggests a more pronounced increase in glucose. Whether this influences wound healing in a clinically relevant way remains to be established. Once assessed, the three studies awaiting classification and two that are ongoing may alter the conclusions of this review.
Conflict of interest statement
Jorinde AW Polderman: none declared.
Violet Farhang‐Razi: none declared.
Susan Van Dieren: none declared.
Peter Kranke.
Consultancy (money paid to author): MSD; Fresenius Kabi.
Grants/grants pending (money to institution): Fresenius Kabi; Premier Research (Acacia Ltd).
Payment for lectures including service on speakers bureaus (money paid to author): Fresenius Kabi; MSD.
None of the companies listed for Dr Kranke's COI have any relation to the current work.
J Hans DeVries.
Consulting fee or honorarium: Johnson.
Board membership (money to institution): Novo Nordisk; Johnson & Johnson; Roche Diagnostics.
Consultancy (money to institution): Sanofi.
Grants/grants pending (money to institution): Dexcom; Senseonics; Novartis; Novo Nordisk; ECHO Therapeutics.
Payment for lectures including service on speakers bureaus (money to institution): Medtronic; Dexcom; Eli Lilly; Roche.
Diagnostics: Novo Nordisk; Sanofi; MSD.
Dr DeVries's institution received research support, fees for advice, and speaker's fees from Abbott Diabetes Care; Dexcom Inc; Eli Lilly; Medtronic; Novo Nordisk; Novartis; Roche Diagnostics; and Sanofi.
None of these bear any relation to the current work. None of the companies listed market products/drugs in this area.
Markus W Hollmann.
Board membership: IARS;
Anesthesia & Analgesia .Consultancy (money to institution): Pfizer; Eurocept; B Braun; ECHO; Fresenius; Merck.
Grants/grants pending (money to institution): ZonMw.
Payment for lectures including service on speakers bureaus (money to institution): Eurocept/Octapharm; Merck; B Braun; Pfizer.
Payment for development of educational presentations (money to institution): Merck.
None of the above declared relationships have influenced this work in that all activities are completely unrelated to the work presented in this review. None of the companies listed market products/drugs in this area.
Benedikt Preckel.
Grants/grants pending (money to institution): European Foundation for the Study of Diabetes; Society of Cardiovascular Anesthesia; Dutch Society of Anesthesia; ZonMw (Dutch Organization for Health Care).
Payment for lectures including service on speakers bureaus (money to institution): Philips Germany; Abbott Netherlands.
None of the companies listed market products/drugs in this area.
Jeroen Hermanides.
Research grants: European Foundation for the Study of Diabetes, European Society for Anaesthesiology. Pending funding (to institution) for investigator‐initiated trial from NovoNordisk. Speaker fee: Eli Lilly.
None of the above declared relationships have influenced this work in that all activities are completely unrelated to the work presented in this review. None of the companies listed market products/drugs in this area.
Figures





Comment in
-
Adverse Effects of Dexamethasone in Surgical Patients.Am J Nurs. 2019 Dec;119(12):19. doi: 10.1097/01.NAJ.0000615756.25925.23. Am J Nurs. 2019. PMID: 31764043
References
References to studies included in this review
-
- Abdelmalak BB, Bonilla A, Mascha EJ, Maheshwari A, Tang WH, You J, et al. Dexamethasone, light anaesthesia, and tight glucose control (DeLiT) randomized controlled trial. British Journal of Anaesthesia 2013;111(2):209‐21. [PUBMED: 23539236] - PubMed
-
- Abdelmalak BB, Bonilla AM, Yang D, Chowdary HT, Gottlieb A, Lyden SP, et al. The hyperglycemic response to major noncardiac surgery and the added effect of steroid administration in patients with and without diabetes. Anesthesia and Analgesia 2013;116(5):1116‐22. [PUBMED: 23558840] - PubMed
-
- Abukawa H, Ogawa T, Kono M, Koizumi T, Kawase‐Koga Y, Chikazu D. Intravenous dexamethasone administration before orthognathic surgery reduces the postoperative edema of the masseter muscle: a randomized controlled trial. Journal of Oral Maxillofacial Surgery 2017;75(6):1257‐62. [PUBMED: 28157491 ] - PubMed
-
- Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. Journal of Arthroplasty 2013;28(8 Suppl):11‐7. [PUBMED: 23937923 ] - PubMed
References to studies excluded from this review
-
- Abbaszadeh M, Khan ZH, Mehrani F, Jahanmehr H. Perioperative intravenous corticosteroids reduce incidence of atrial fibrillation following cardiac surgery: a randomized study. Revista Brasileira De Cirurgia Cardiovascular 2012;27(1):18‐23. [PUBMED: 22729297] - PubMed
-
- Bianchin A, Luca A, Caminiti A. Postoperative vomiting reduction after laparoscopic cholecystectomy with single dose of dexamethasone. Minerva Anestesiologica 2007;73(6):343‐6. [PUBMED: 17589423] - PubMed
-
- Coloma M, Duffy LL, White PF, Kendall Tongier W, Huber PJ. Dexamethasone facilitates discharge after outpatient anorectal surgery. Anesthesia and Analgesia 2001;92(1):85‐8. [PUBMED: 11133606] - PubMed
-
- Halvorsen P, Raeder J, White PF, Almdahl SM, Nordstrand K, Saatvedt K, et al. The effect of dexamethasone on side effects after coronary revascularization procedures. Anesthesia and Analgesia 2003;96(6):1578‐83, table of contents. [PUBMED: 12760978] - PubMed
References to studies awaiting assessment
-
- Ko‐Iam W, Sandhu T, Paiboonworachat S, Pongchairerks P, Junrungsee S, Chotirosniramit A, et al. Metoclopramide versus its combination with dexamethasone in the prevention of postoperative nausea and vomiting after laparoscopic cholecystectomy: a double‐blind randomized controlled trial. Journal of the Medical Association of Thailand 2015;98(3):265‐72. [PUBMED: 25920297] - PubMed
-
- Simogai M, Kayo R. Prevention of postoperative nausea and vomiting associated with postoperative fentanyl infusion by dexamethasone and combined diphenhydramine and diprophylline preparation (Travelmin). [Japanese]. Medical Journal of Minami Osaka Hospital 2016;63(1):17‐20.
References to ongoing studies
-
- Kitcharanant N. Effects of perioperative intravenous dexamethasone on the severity of persistent postsurgical pain after total knee arthroplasty: a prospective, randomized, double‐blind, placebo‐controlled trial. ClinicalTrials.gov: NCT02760459.
-
- Sidhu J. Perioperative ADministration of Dexamethasone and Infection trial. www.paddi.org.au (accessed 16 August 2018).
-
- Wang DX. Dexamethasone, flurbiprofen, axetil and delirium after lung cancer surgery. ClinicalTrials.gov: NCT02300600. [Clinicaltrials.gov NCT03200600]
Additional references
-
- Althumairi AA, Canner JK, Gearhart SL, Safar B, Sacks J, Efron JE. Predictors of perineal wound complications and prolonged time to perineal wound healing after abdominoperineal resection. World Journal of Surgery 2016;40(7):1755‐62. [PUBMED: 26908238 ] - PubMed
-
- Assante J, Collins S, Hewer I. Infection associated with single‐dose dexamethasone for prevention of postoperative nausea and vomiting: a literature review. AANA Journal 2015;83(4):281‐8. [PUBMED: 26390747] - PubMed
-
- Bartlett R, Hartle AJ. Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia 2013;68(9):892‐6. [PUBMED: 23848377] - PubMed
-
- Bolac CS, Wallace AH, Broadwater G, Havrilesky LJ, Habib AS. The impact of postoperative nausea and vomiting prophylaxis with dexamethasone on postoperative wound complications in patients undergoing laparotomy for endometrial cancer. Anesthesia and Analgesia 2013;116(5):1041‐7. [PUBMED: 23337415 ] - PubMed
-
- Busti AJ, Hooper JS, Amaya CJ, Kazi S. Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy 2005;25(11):1566‐91. [PUBMED: 16232020] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

