Using hyperpolarised NMR and DFT to rationalise the unexpected hydrogenation of quinazoline to 3,4-dihydroquinazoline
- PMID: 30152480
- PMCID: PMC6136267
- DOI: 10.1039/c8cc04826f
Using hyperpolarised NMR and DFT to rationalise the unexpected hydrogenation of quinazoline to 3,4-dihydroquinazoline
Abstract
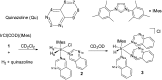
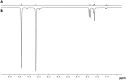
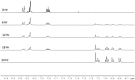
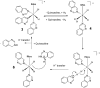
PHIP and SABRE hyperpolarized NMR methods are used to follow the unexpected metal-catalysed hydrogenation of quinazoline (Qu) to 3,4-dihydroquinazoline as the sole product. A solution of [IrCl(IMes)(COD)] in dichloromethane reacts with H2 and Qu to form [IrCl(H)2(IMes)(Qu)2] (2). The addition of methanol then results in its conversion to [Ir(H)2(IMes)(Qu)3]Cl (3) which catalyses the hydrogenation reaction. Density functional theory calculations are used to rationalise a proposed outer sphere mechanism in which (3) converts to [IrCl(H)2(H2)(IMes)(Qu)2]Cl (4) and neutral [Ir(H)3(IMes)(Qu)2] (6), both of which are involved in the formation of 3,4-dihydroquinazoline via the stepwise transfer of H+ and H-, with H2 identified as the reductant. Successive ligand exchange in 3 results in the production of thermodynamically stable [Ir(H)2(IMes)(3,4-dihydroquinazoline)3]Cl (5).
Figures
References
-
- Guenther U. L., Modern NMR Methodology, 2013, vol. 335, pp. 23–69. - PubMed
-
- Oros A. M., Shah N. J. Phys. Med. Biol. 2004;49:R105–R153. - PubMed
-
- Viale A., Reineri F., Santelia D., Cerutti E., Ellena S., Gobetto R., Aime S. Q. J. Nucl. Med. Mol. Imaging. 2009;53:604–617. - PubMed
-
- Lee D., Monin G., Duong N. T., Lopez I. Z., Bardet M., Mareau V., Gonon L., De Paepe G. J. Am. Chem. Soc. 2014;136:13781–13788. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

