Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation
- PMID: 30153002
- PMCID: PMC6329606
- DOI: 10.1021/jacs.8b06458
Mechanistic Interrogation of Co/Ni-Dual Catalyzed Hydroarylation
Abstract
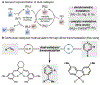
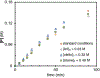
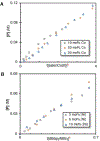
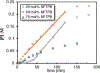
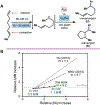
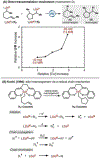
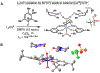
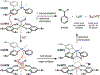
Cobalt/nickel-dual catalyzed hydroarylation of terminal olefins with iodoarenes builds complexity from readily available starting materials, with a high preference for the Markovnikov (branched) product. Here, we advance a mechanistic model of this reaction through the use of reaction progress kinetic analysis (RPKA), radical clock experiments, and stoichiometric studies. Through exclusion of competing hypotheses, we conclude that the reaction proceeds through an unprecedented alkylcobalt to nickel direct transmetalation. Demonstration of catalytic alkene prefunctionalization, via spectroscopic observation of an organocobalt species, distinguishes this Csp2-Csp3 cross-coupling method from a conventional transmetalation process, which employs a stoichiometric organometallic nucleophile, and from a bimetallic oxidative addition of an organohalide across nickel, described by radical scission and subsequent alkyl radical capture at a second nickel center. A refined understanding of the reaction leads to an optimized hydroarylation procedure that excludes exogenous oxidant, demonstrating that the transmetalation is net redox neutral. Catalytic alkene prefunctionalization by cobalt and engagement with nickel catalytic cycles through direct transmetalation provides a new platform to merge these two rich areas of chemistry in preparatively useful ways.
Figures

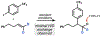
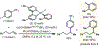
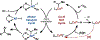
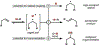



References
-
- Miyaura N; Suzuki A Chem. Rev 1995, 95, 2457–2483.
-
- For seminal reports on dual-catalytic transmetalations, see Cu/Pd: (a) Sonogashira K; Tohda Y; Hagihara N Tetrahedron Lett 1975, 16, 4467–4470.
- Gooßen LJ; Deng G; Levy LM Science 2006, 313, 662–664. Cu/Ni: - PubMed
- Semba K; Ohtagaki Y; Nakao Y Org. Lett 2016, 18, 3956–3959. Pd/Ni: - PubMed
- Ackerman LKG; Lovell MM; Weix DJ Nature 2015, 524, 454–457. Rh/Pd: - PMC - PubMed
- Sawamura M; Sudoh M; Ito YJ Am. Chem. Soc 1996, 118, 3309–3310. Au/Pd:
- Shi Y; Roth KE; Ramgren SD; Blum SA J. Am. Chem. Soc. 2009, 131, 18022–18023. - PubMed
- García-Dominguez P; Nevado CJ Am. Chem. Soc 2016, 138, 3266–3269. Fe/Pd: - PubMed
- Chen B; Ma S Chem. - Eur. J 2011, 17, 754–757. Ni/Cr: - PubMed
- Fürstner A; Shi NJ Am. Chem. Soc 1996, 118, 2533–2534. V/Pd:
- Trost BM; Luan XJ Am. Chem. Soc 2011, 133, 1706–1709. - PubMed
-
- Green SA; Matos JLM; Yagi A; Shenvi RA J. Am. Chem. Soc 2016, 138, 12779–12782. - PubMed
-
- (a) Iwasaki K; Wan KK; Oppedisano A; Crossley SWM; Shenvi RA J. Am. Chem. Soc 2014, 136, 1300–1303. - PMC - PubMed
- Crossley SWM; Martinez RM; Guevera-Zuluaga S; Shenvi RA Org. Lett 2016, 18, 2620–2623. - PMC - PubMed
- Obradors C; Martinez RM; Shenvi RA J. Am. Chem. Soc 2016, 138, 4962–4971. - PMC - PubMed
- Crossley SWM; Barabe,´ F.; Shenvi, R. A. J. Am. Chem. Soc 2014, 136, 16788–16791. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

