The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction
- PMID: 30153110
- PMCID: PMC6205385
- DOI: 10.1172/JCI95945
The two-pore domain potassium channel TREK-1 mediates cardiac fibrosis and diastolic dysfunction
Abstract
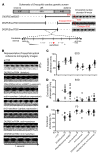
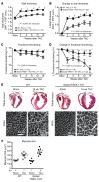
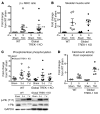
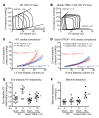
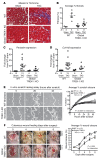
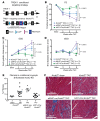
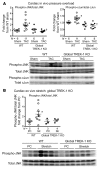
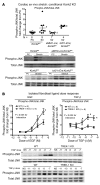
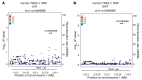
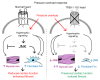
Cardiac two-pore domain potassium channels (K2P) exist in organisms from Drosophila to humans; however, their role in cardiac function is not known. We identified a K2P gene, CG8713 (sandman), in a Drosophila genetic screen and show that sandman is critical to cardiac function. Mice lacking an ortholog of sandman, TWIK-related potassium channel (TREK-1, also known Kcnk2), exhibit exaggerated pressure overload-induced concentric hypertrophy and alterations in fetal gene expression, yet retain preserved systolic and diastolic cardiac function. While cardiomyocyte-specific deletion of TREK-1 in response to in vivo pressure overload resulted in cardiac dysfunction, TREK-1 deletion in fibroblasts prevented deterioration in cardiac function. The absence of pressure overload-induced dysfunction in TREK-1-KO mice was associated with diminished cardiac fibrosis and reduced activation of JNK in cardiomyocytes and fibroblasts. These findings indicate a central role for cardiac fibroblast TREK-1 in the pathogenesis of pressure overload-induced cardiac dysfunction and serve as a conceptual basis for its inhibition as a potential therapy.
Keywords: Cardiology; Cardiovascular disease; Fibrosis; Heart failure; Muscle Biology.
Conflict of interest statement
Figures
References
-
- Schmidt C, et al. Stretch-activated two-pore-domain (K2P) potassium channels in the heart: Focus on atrial fibrillation and heart failure. Prog Biophys Mol Biol. 2017;130(Pt B):233–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

