The Effect of a Head-mounted Low Vision Device on Visual Function
- PMID: 30153237
- PMCID: PMC6133226
- DOI: 10.1097/OPX.0000000000001262
The Effect of a Head-mounted Low Vision Device on Visual Function
Abstract
Significance: Head-mounted low vision devices have received considerable attention in recent years owing to rapidly developing technology, facilitating ease of use and functionality. Systematic clinical evaluations of such devices remain rare but are needed to steer future device development.
Purpose: The purpose of this study was to investigate, in a multicenter prospective trial, the short- and medium-term effects of a head-worn vision enhancement device (eSight Eyewear).
Methods: Participants aged 13 to 75 years with stable vision (distance acuity, 20/60 to 20/400; visual field diameter >20°) were recruited across six sites. Data were collected at baseline (no device), at fitting (with device), and after 3 months of everyday use. Outcome measures were visual ability measured by the Veterans Affairs Low Vision Visual Functioning Questionnaire 48, distance acuity (Early Treatment Diabetic Retinopathy Study), reading performance (MNREAD chart), contrast sensitivity (MARS chart), face recognition, and a modified version of the Melbourne Low Vision Activities of Daily Living (ADL) Index.
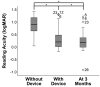
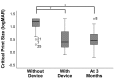
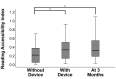
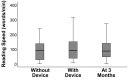
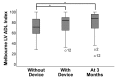
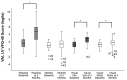
Results: Among the 51 participants, eSight introduction immediately improved distance acuity (0.74 ± 0.28 logMAR), contrast sensitivity (0.57 ± 0.53 log units), and critical print size (0.52 ± 0.43 logMAR), all P < .001, without any further change after 3 months; reading acuity improved at fitting (0.56 ± 0.35 logMAR) and by one additional line after 3 months, whereas reading speed only slightly increased across all three time points. The Melbourne ADL score and face recognition improved at fitting (P < .01) with trends toward further improvement at 3 months. After 3 months of use, Veterans Affairs Low Vision Visual Functioning Questionnaire 48 person measures (in logits) improved: overall, 0.84, P < .001; reading, 2.75, P < .001; mobility, 0.04, not statistically significant; visual information, 1.08, P < .001; and visual motor, 0.48, P = .02.
Conclusions: eSight introduction yields immediate improvements in visual ability, with face recognition and ADLs showing a tentative benefit of further use. Overall, visual ability, reading, and visual information showed greatest benefit with device use. Further studies need to examine benefits of practice and training and possible differential effects of underlying pathology or baseline vision.
Conflict of interest statement
Figures





References
-
- Wolffsohn JS, Peterson RC. A Review of Current Knowledge on Electronic Vision Enhancement Systems for the Visually Impaired. Ophthalmic Physiol Opt 2003;23:35–42. - PubMed
-
- Longley C, Whitaker D. Google Glass Glare: Disability Glare Produced by a Head-mounted Visual Display. Ophthalmic Physiol Opt 2016;36:167–73. - PubMed
-
- Lin CS, Yang HJ, Lay YL, et al. Design and Evaluation of a Public Installation of an Eye-gaze System. Assist Technol 2011;23:187–98. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

